Preparation method of trelagliptin
A methyl and dioxo technology, applied in the direction of organic chemistry and the like, can solve the problems of low selectivity of troxagliptin, easy generation of by-products, etc., and achieve novel synthetic route, great implementation value, social and economic benefits, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 6.42g of 3-methyl-6-chlorouracil and 10.27g of 2-cyano-5-fluorobenzyl bromide in 40ml of ethyl acetate, add 8.4ml of triethylamine, stir and raise the temperature to 80°C, and react for 2 hours . Cool down to room temperature, recover the solvent, extract with water and ethyl acetate, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent. The residue was purified by column chromatography to obtain 9.54 g of product with a yield of 81.2% and a purity of 99.7%.
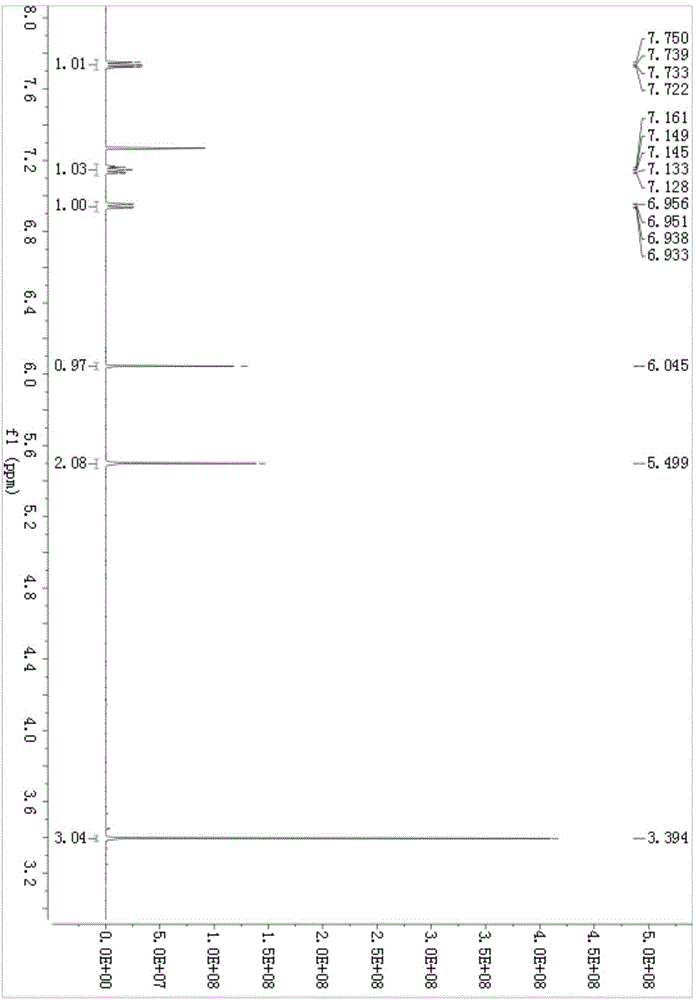
[0030] 1 H NMR (500MHz, CDCl 3 )δ:7.75(dd,J 1 =5.5Hz,J 2 =8.5Hz,1H),7.16-7.12(m,1H),7.45(t,J=7.5Hz,1H),6.95(dd,J 1 =2.5Hz,J 2 =9.0Hz, 1H), 6.04(s, 1H), 5.49(s, 2H), 3.39(s, 3H). (See attached figure 1 )
Embodiment 2
[0032] Dissolve 32.1 g of 3-methyl-6-chlorouracil and 47.1 g of 2-cyano-5-fluorobenzyl bromide in 200 ml of N,N-dimethylformamide (DMF), add 56 ml of triethylamine, stir and The temperature was raised to 100°C, and the reaction was carried out for 2 hours. After cooling down to room temperature, adding water and ethyl acetate for extraction, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was recovered. The residue was purified by column chromatography to obtain 49.3 g of the product with a yield of 83.9% and a purity of 99.6%.
[0033] (2) (R)-tert-butyl-1-(3-(2-isocyano-5-fluoro-benzyl)-1-methyl-2,6-dioxo-1,2,3,6 Preparation of -tetrahydropyrimidin-4-yl)piperidin-3-ylcarbamate (Ⅵ)
Embodiment 3
[0035] 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile 58.7g and (R Dissolve 48g of )-3-Boc-aminopiperidine in 500ml of toluene, add 55g of potassium carbonate and 1.0g of tetrabutylammonium bromide, stir vigorously and raise the temperature to 100°C, and react for 24 hours. Cool down to room temperature, recover the solvent, add water and ethyl acetate for extraction, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, recover the solvent to obtain a crude product, recrystallize with ethyl acetate and petroleum ether to obtain 84.2 g of off-white solid, Yield 92.0%, purity 99.3%.
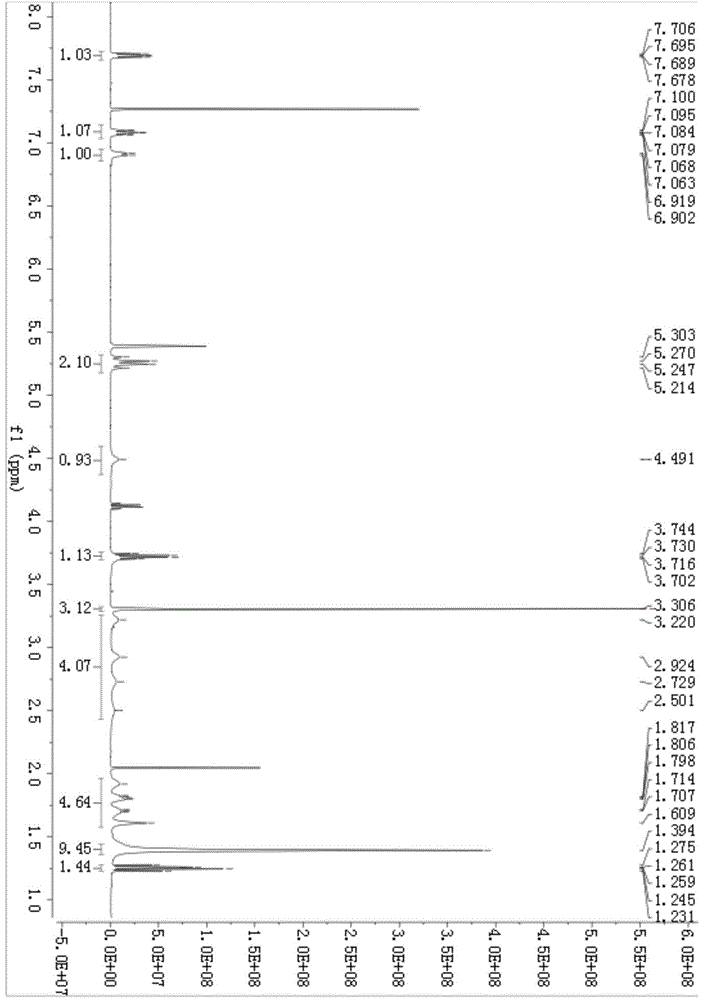
[0036] 1 H NMR (500MHz, CDCl 3 ):7.70(dd,J 1 =5.5Hz,J 2 =8.5Hz,1H),7.10-7.06(m,1H),6.91(d,J=8.5Hz,1H),5.30(dd,J 1 =16.5Hz,J 2 =28.0Hz,2H),4.49(s,1H),3.74-3.70(m,1H),3.30(s,3H),3.22-2.50(m,4H),1.91-1.60(m,4H),1.39( s,9H),1.27-1.23(m,1H).(see attached figure 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com