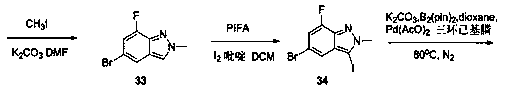
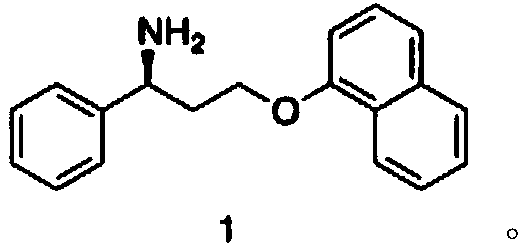
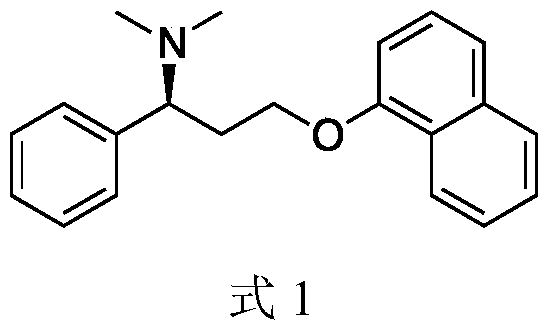
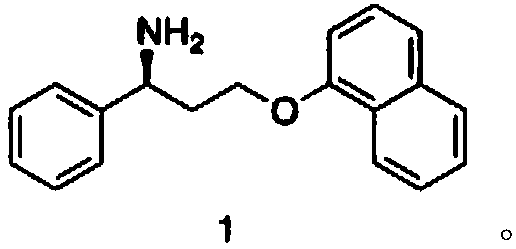
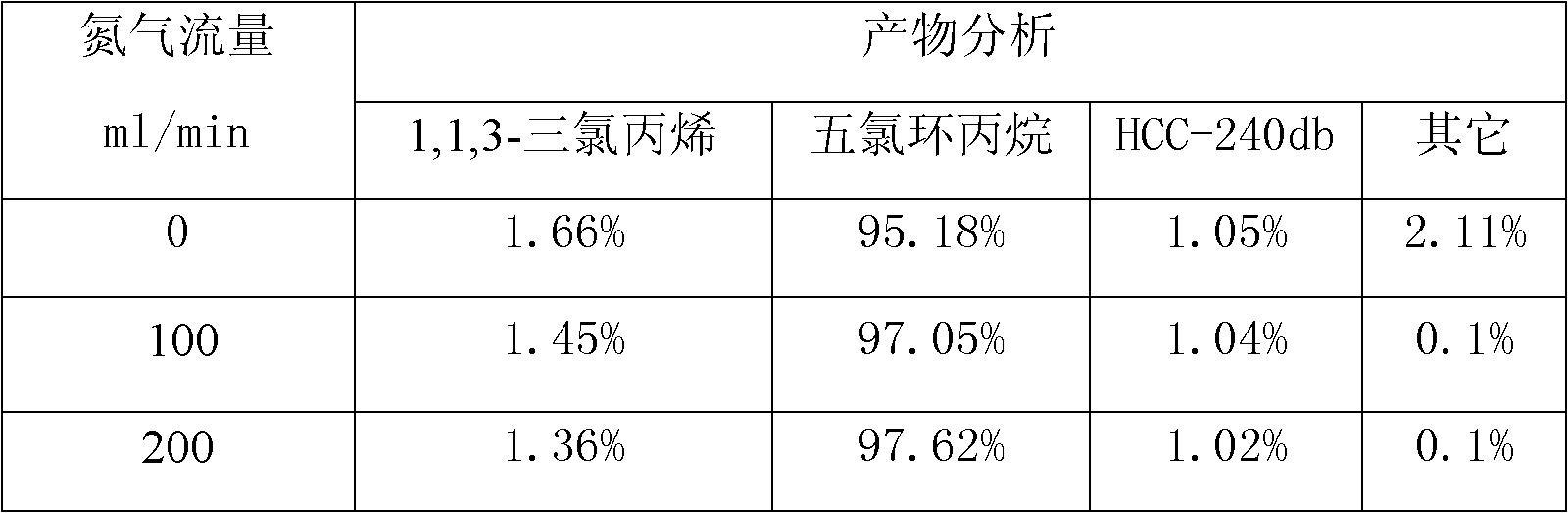
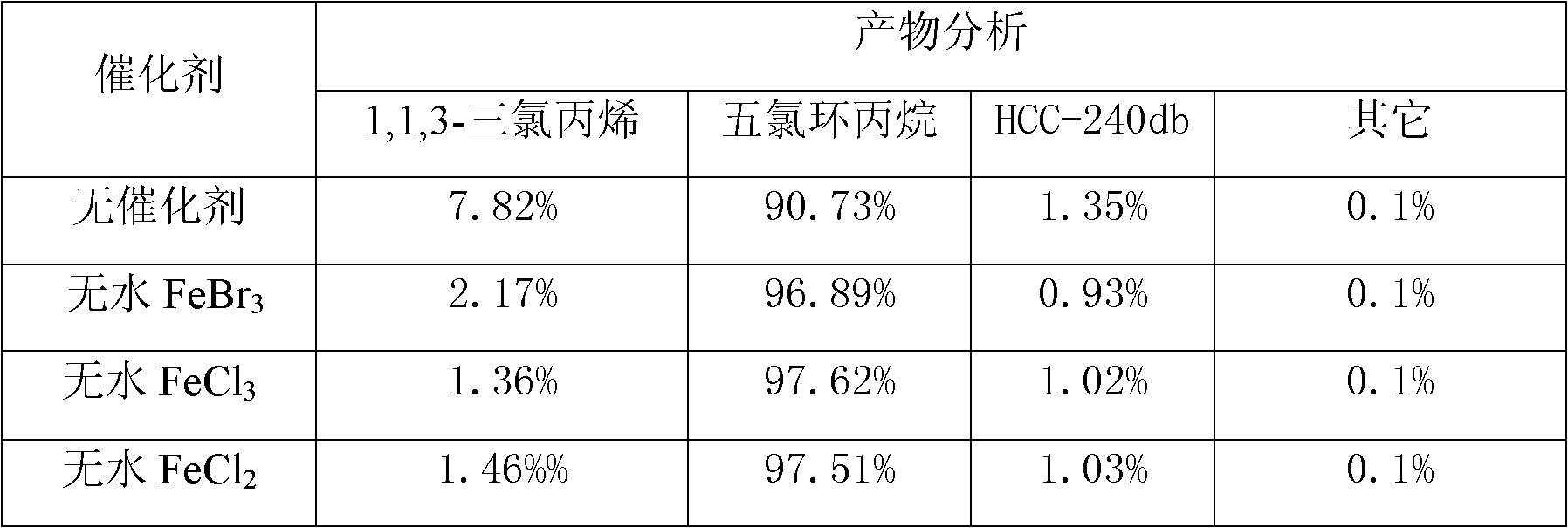
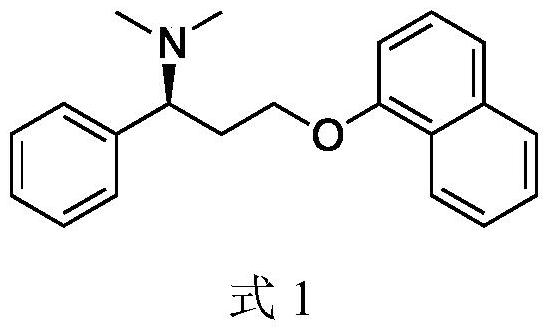
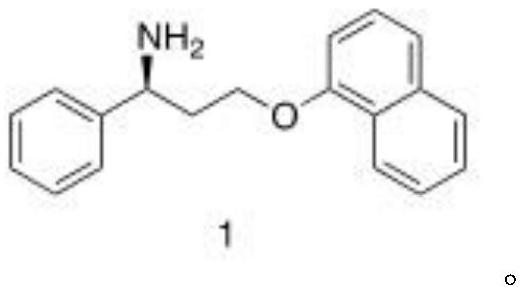
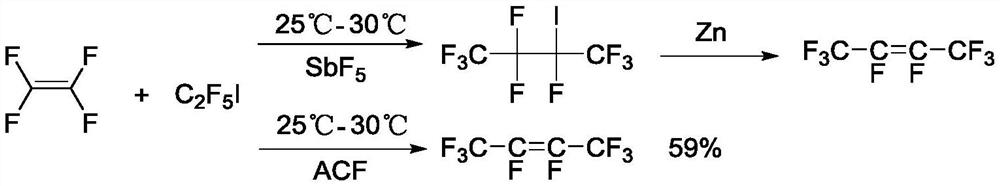
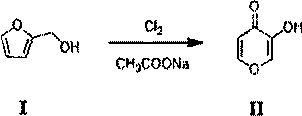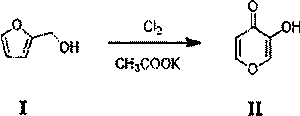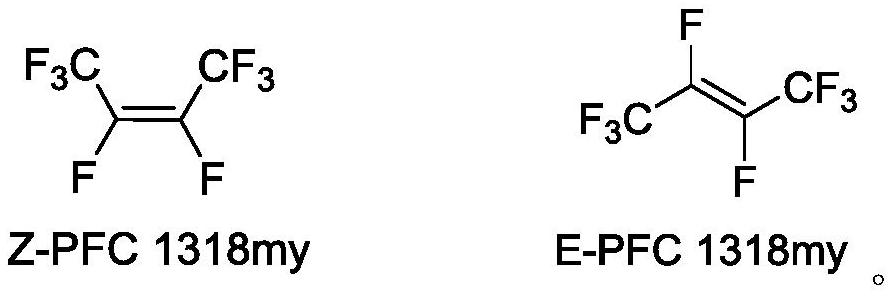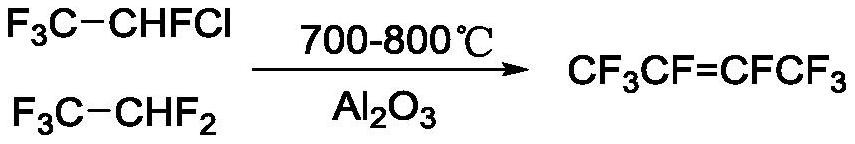Patents
Literature
46results about How to "Novel synthetic route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-aroyl-1,8-naphthalimide compound and preparation method and use thereof
InactiveCN104003935AReduce manufacturing costNovel synthetic routeOrganic chemistryMaterial analysis by observing effect on chemical indicatorAcyl groupOrganosolv
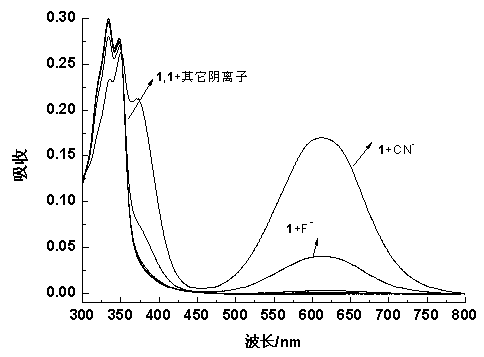
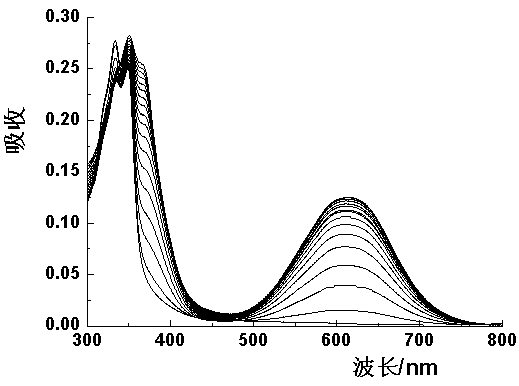
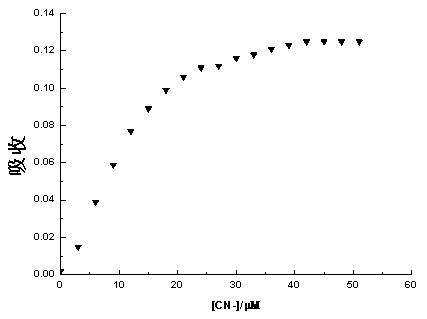
The invention discloses a 4-aroyl-1,8-naphthalimide compound and a preparation method and use thereof, wherein the 4-aroyl-1,8-naphthalimide compound has a structural formula as shown in the specification, R1 is C1-C10 straight chain or branched chain alkyl; and R2 is phenyl, naphthyl, biphenylyl, substituted phenyl, quinary or senary heteroaryl or benzo quinary or senary heteroaryl. The preparation method is as follows: a 4 bromo-1,8-naphthalimide compound is used as a raw material to react with substituted phenylacetonitrile or aromatic ring acetonitrile in an organic solvent in the presence of an alkali catalyst to obtain a 4-aryl acetonitrile-1,8-naphthalimide compound, and then the 4-aroyl-1,8-naphthalimide compound is obtained in the effects of fluoride ions or cyanide ions. The 4-aryl acetonitrile-1, 8-naphthalimide compound is used as a color or fluorescence sensor for detection of the fluoride ions or cyanide ions, and has high sensitivity and high selectivity during identifying of the cyanide ions in a mixed solvent.
Owner:SHANGHAI INST OF TECH
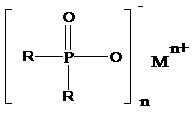
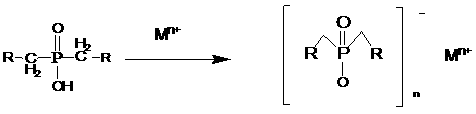
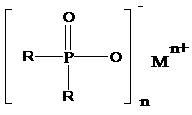
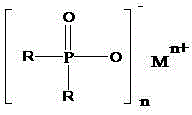
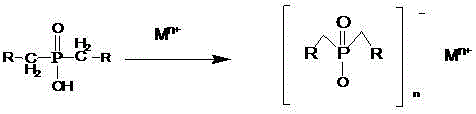
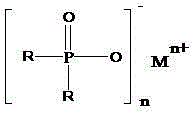
Salts of dialkylphosphinic acid, preparation method and application
ActiveCN102718798AAvoid dangerAvoid it happening againGroup 5/15 element organic compoundsPhosphinic AcidsAqueous solution
The invention discloses a preparation method and an application of salts of dialkylphosphinic acid, the salts of dialkylphosphinic acid has a structure shown in a formula (I). The preparation method of the salts of dialkylphosphinic acid comprises the following steps: hypophosphorous acid or hypophosphites are reacted with aldehyde in an aqueous solution to obtain di(alpha-hydroxy)alkyl hypophosphorous acid, hydroxy is reduced through HI / P to obtain an dialkylphosphinic acid solution, and the dialkylphosphinic acid solution and metal salt are reacted to obtain the corresponding salts of dialkylphosphinic acid. The preparation method of salts of dialkylphosphinic acid has the advantages of high yield, simple synthesis process and convenient operation, and substantially reduces production cost, simplifies production equipment and enhances the security of production process.
Owner:KINGFA SCI & TECH CO LTD +1
Preparation method of trelagliptin
InactiveCN104961726AControl contentAvoid it happening againOrganic chemistrySocial benefitsChemical synthesis
The invention belongs to the field of chemical synthesis of medicines, and particularly relates to a preparation method of trelagliptin. A synthesis path of the trelagliptin is shown in the specification. The trelagliptin which is protected by Boc is synthesized by (R)-3-Boc-aminopiperidines, the trelagliptin is obtained through hydrolysis deprotection, by-products produced by reaction on amidogen can be avoided, the synthesis path is novel, technological conditions are reasonable, the type and the content of impurities in the trelagliptin can be controlled effectively, a technology is easy to operate, and the yield is high. Moreover, the preparation method of the trelagliptin is suitable to be produced industrially and has high application value, high social benefit and high economic benefit.
Owner:ZHEJIANG YONGNING PHARMA
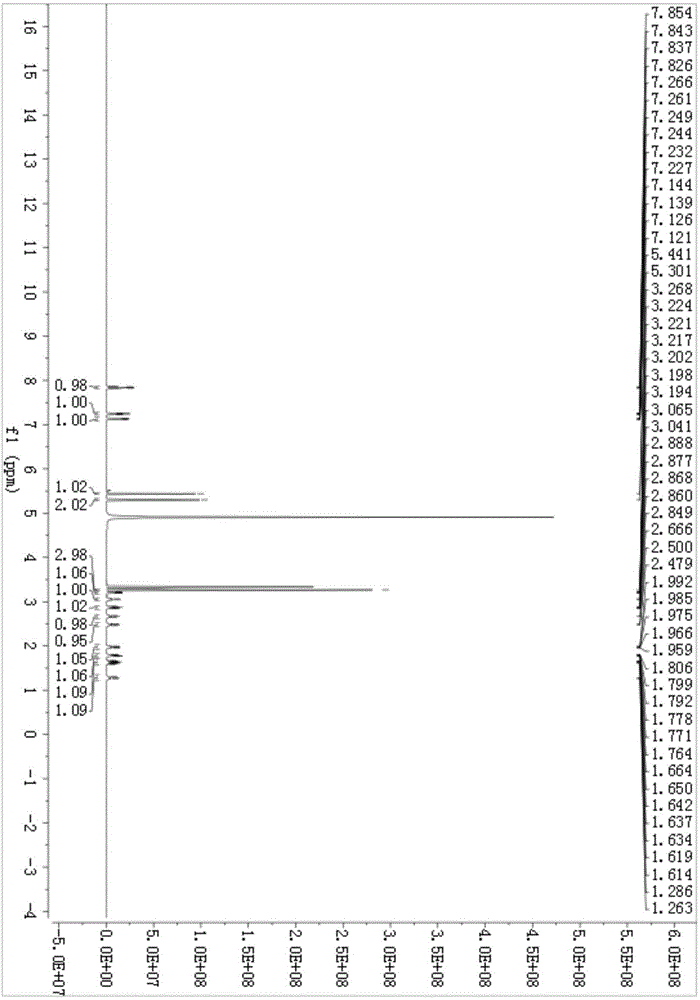
Synthesis method of 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid
PendingCN109438405ALower catalyst costsNovel synthetic routeOrganic chemistryBulk chemical productionFurfuryl alcoholBatch production
The invention discloses a synthesis method of 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid. The synthesis method takes furfuryl alcohol as a starting raw material and four-step reaction including rearrangement, addition, hydroxyl protection and oxidization is carried out; the total mol yield of a synthesis route is greater than 32 percent; the synthesis method has the characteristics of relatively moderate reaction conditions and the like, and the yield and purity of the 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid are remarkably improved; meanwhile, the synthesis method has the advantagesof detailed technological operation steps, specific parameters, controllable conditions and stable technology, and can realize industrial large-batch production.
Owner:RAFFLES PHAMRMATECH CO LTD
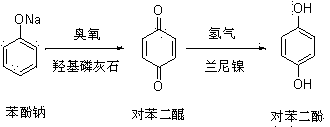
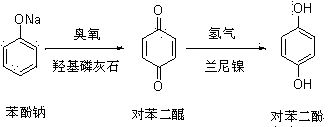
Hydroquinone synthetic method
InactiveCN103420803ANovel synthetic routeThe process is simple and feasibleOrganic chemistryOrganic compound preparationQuinoneHydrogen
The invention discloses a hydroquinone synthetic method. The method comprises the steps that sodium phenate is reacted with ozone on the condition that catalyst hydroxyapatite exists, and benzene quinone is obtained after reaction liquid water steam is distilled; hydroquinone is obtained after hydrogen is added into the benzene quinone on the condition that catalyst raney nickel exists. The hydroquinone synthetic method is simple in synthetic route, novel, simple in technology, high in yield and purity of products, incapable of influencing the environment, and suitable for industrial production. Moreover, catalyst is cheap and can be obtained easily.
Owner:CHONGQING TECH & BUSINESS UNIV
Asymmetric cyclobutane derivatives, preparation method and applications thereof
ActiveCN105294639AGood immunosuppressive activityNovel synthetic routeOrganic active ingredientsOrganic chemistryChemical synthesisChemical reaction
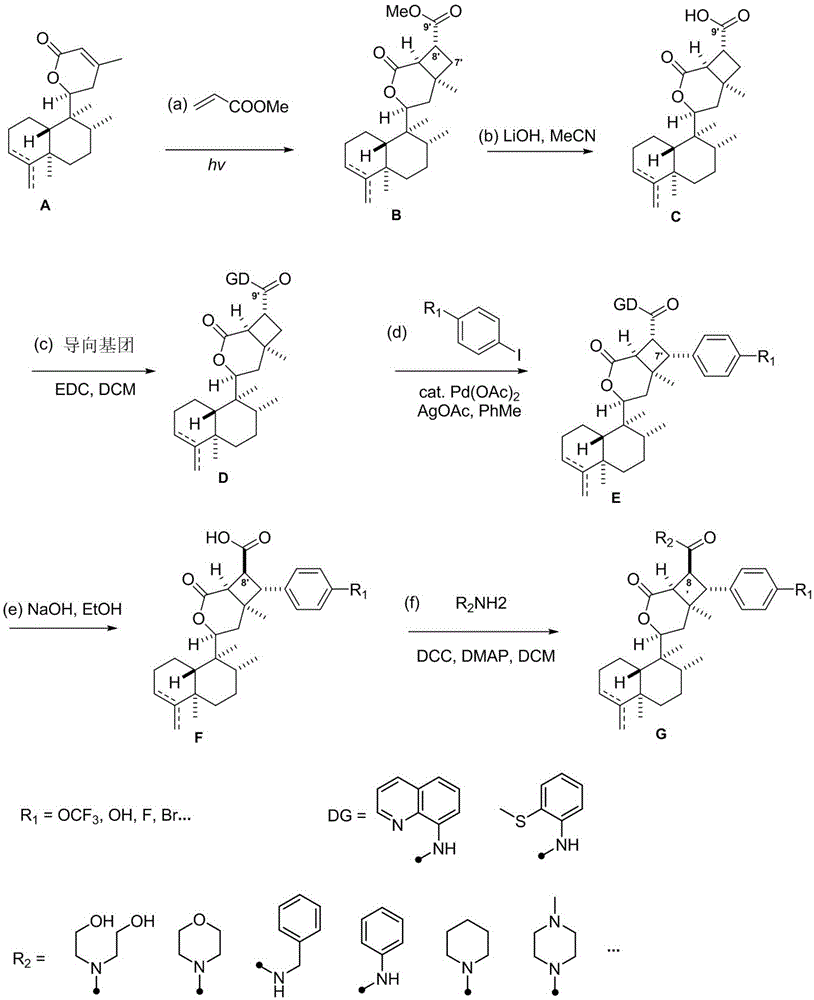
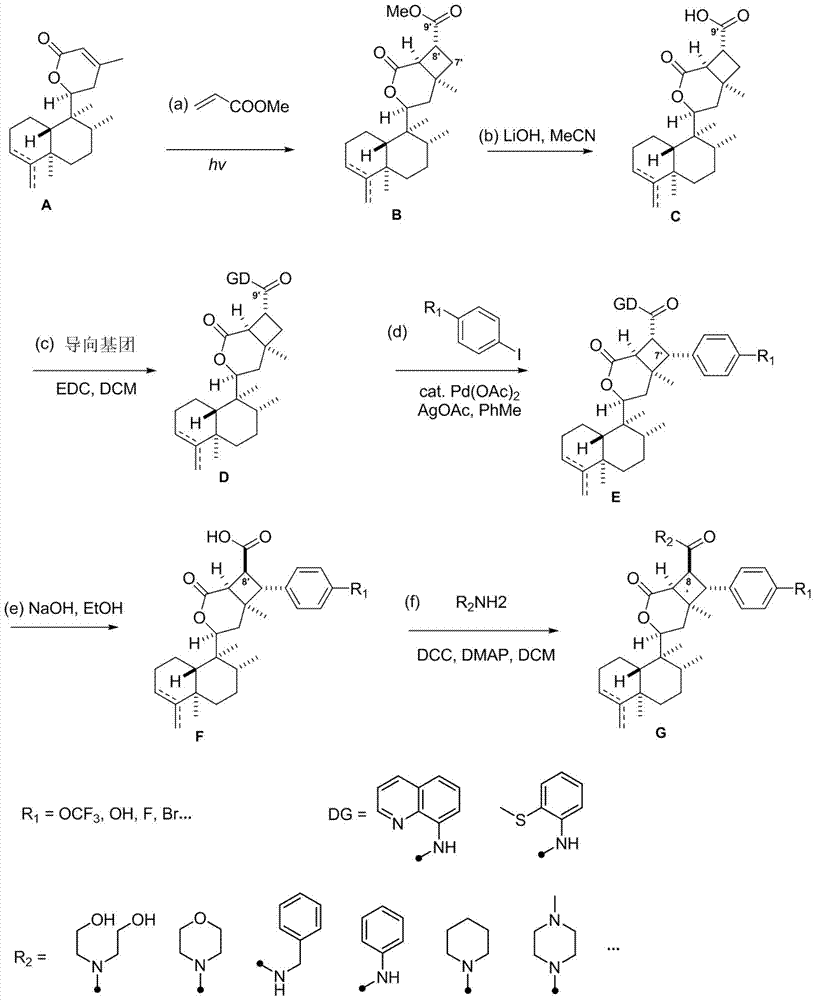
The invention discloses asymmetric cyclobutane derivatives (synthetic derivatives G of rabdosia scoparia lactones), which are named as scopariusicide derivatives, a pharmaceutical composition taking the asymmetric cyclobutane derivatives as the active component, a preparation method of asymmetric cyclobutane derivatives, and an application of asymmetric cyclobutane derivatives in preparation of immuno-suppressive drugs. According to the preparation, scopariusicides, which is derived from pharmaceutical plants, is taken as the template; a precursor compound ent-clerodane diterpene A is taken as the initial raw material, a series of artificial analogues are obtained through six steps of chemical synthesis; the basic structure of the derivatives has a characteristic that ent-clerodane hetero-diterpene has an asymmetric cyclobutane segment, the side chain and aromatic ring of the derivatives may have different substituents, and thus there are many kinds of derivatives. The compounds are synthesized for the first time, the synthesis route is novel, and the derivatives have potential bioactivity, can be used as the lead compounds of immuno-suppressive drugs, and have a very important research value and application prospect.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Biosynthesis method of atomoxetine intermediate and carbonyl reductase
ActiveCN112359028AHigh catalytic efficiencyImprove solubilityBacteriaMicroorganism based processesCarbonyl groupCarbonyl Reductase
The invention relates to the technical field of enzyme engineering and particularly relates to a biosynthesis method of a atomoxetine intermediate and carbonyl reductase, the carbonyl reductase producing strain is Escherichia coli TM1908, and the preservation number of the strain is CCTCC NO: M2019714. In the presence of a coenzyme, a coenzyme circulating enzyme, a cosolvent and a buffer solution,the carbonyl reductase is catalyzed and converted into a atomoxetine intermediate compound I. The invention further discloses a preparation method of the atomoxetine intermediate compound I. The synthesis method provided by the invention solves a problem of chemical catalysis of low-chirality products in the prior art, reduces production cost and reduces pollution. Meanwhile, the invention provides a new catalytic substrate for the preparation of the important chiral intermediate of the atomoxetine.
Owner:JIANGSU ALPHA PHARM CO LTD
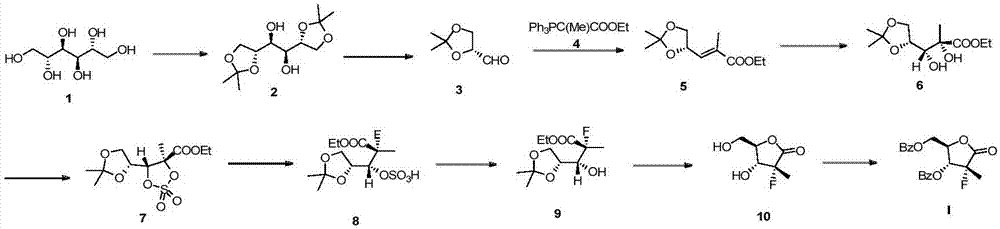
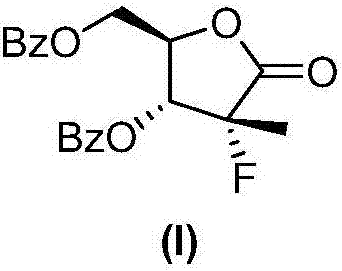
Preparation method of sofosbuvir intermediate
InactiveCN107573304ANovel synthetic routeShort stepsOrganic chemistryIntermediate structureSofosbuvir
The invention discloses a preparation method of a sofosbuvir intermediate of (2R)-2-deoxy-2-fluoro-2-methyl-D-erythro-pentonic acid-g-lactone 3,5-dibenzoate. The intermediate structure corresponds toa formula I shown as the description. The method comprises the step that the sofosbuvir intermediate shown as the formula I is finally obtained by using (D2) (S)-(+)-4-phenyl-2-oxazolidinone of a compound shown as a formula II as a raw material through six-step reaction of condensation, fluorination, Adol addition, oxidation cyclization, isomerization and benzoylation. The preparation method hasthe advantages that the raw materials are cheap and can be easily obtained; the reaction conditions are mild; the route is short; the yield is high; three wastes are few; the pollution is little; thepreparation method is suitable for industrial production.
Owner:PHARMA SHANGHAI +1
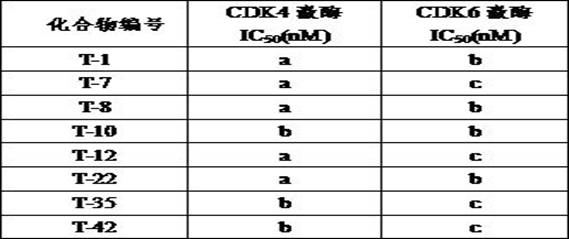
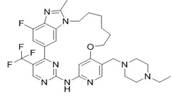
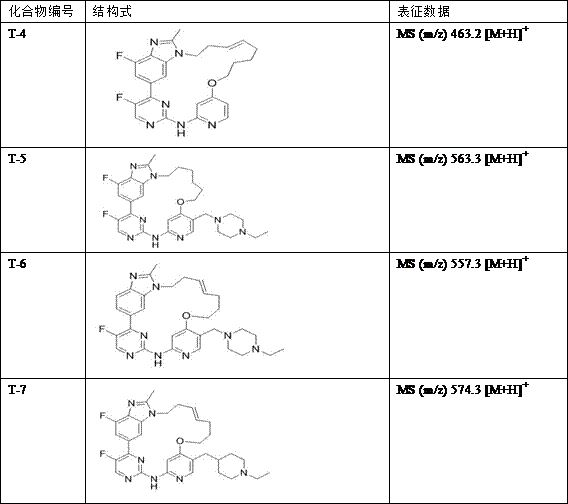
Cyclic aminopyrimidine derivatives as well as kinase inhibition activity and application thereof
ActiveCN111269245AGood clinical valueNovel synthetic routeOrganic chemistryAntineoplastic agentsPharmaceutical medicinePerylene derivatives
The invention discloses cyclic aminopyrimidine derivatives as well as kinase inhibition activity and application thereof, and particularly discloses cyclic aminopyrimidine derivatives having structures as shown in a general formula (I) and pharmaceutically acceptable salts, esters or solvent compounds thereof. The derivatives can inhibit the activity of kinase, are inhibitors for a variety of kinases, can be more widely applied to treatment of cancers, and have huge clinical application prospects. The general formula (I) is shown in the specification.
Owner:HANGZHOU BIO CREATIVITY PHARM TECH CO LTD
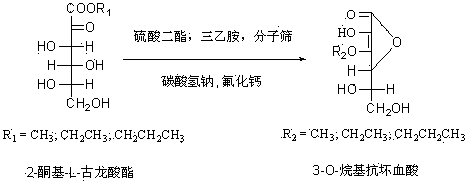
Preparation method for 3-O-alkyl ascorbic acid
InactiveCN103467420ASimple post-processingNovel synthetic routeOrganic chemistrySodium bicarbonateMolecular sieve
The invention discloses a preparation method for 3-O-alkyl ascorbic acid. The preparation method comprises the steps that under protection of nitrogen, 2-keto-L-gulonic acid ester reacts with sulfuric acid diester at the presence of catalyst molecular sieves, and an obtained intermediate under the action of sodium bicarbonate and calcium fluoride generates the 3-O-alkyl ascorbic acid. According to the preparation method, a synthetic route is simple, a synthetic method is novel, a process is easy and convenient to achieve, postprocessing is simple, the yield and purity of products are high, catalysts are low in price and easy to obtain, the environment is not affected, and the preparation method is suitable for industrial production.
Owner:CHONGQING TECH & BUSINESS UNIV
4-phenoxybenzoic acid synthesis method
InactiveCN110407693ANovel synthetic routeSuitable for industrial productionOrganic compound preparationCarboxylic acid esters preparationChemical industrySodium Phenolate
The invention relates to the technical field of chemical industry synthesis, and particularly discloses a 4-phenoxybenzoic acid synthesis method, which comprises: carrying out a mixing heating reaction on phenol as a raw material, sodium hydroxide and water to obtain a sodium phenolate solution; slowly adding the sodium phenolate solution into a mixture of tetrahydronaphthalene and p-chlorobenzoicacid in a dropwise manner while heating, and carrying out a thermal insulation reaction; after completing the reaction, cooling the reaction liquid, filtering, washing the obtained solid, filtering,mixing by adding clear water, adjusting the pH value, and filtering to obtain crude and wet 4-phenoxybenzoic acid; and dissolving the crude and wet 4-phenoxybenzoic acid in ethanol, adsorbing with active carbon, filtering, cooling, and filtering to obtain the product. According to the present invention, the process route is simple and easy to operate, the reaction can be completed in one step, thestarting raw material is simple and cheap, and is easy to obtain, the generated waste water is less, and the method is suitable for industrial production, and is a completely-new synthesis route.
Owner:CHENGWU CHENHUI ENVIRONMENTAL PROTECTION TECH CO LTD
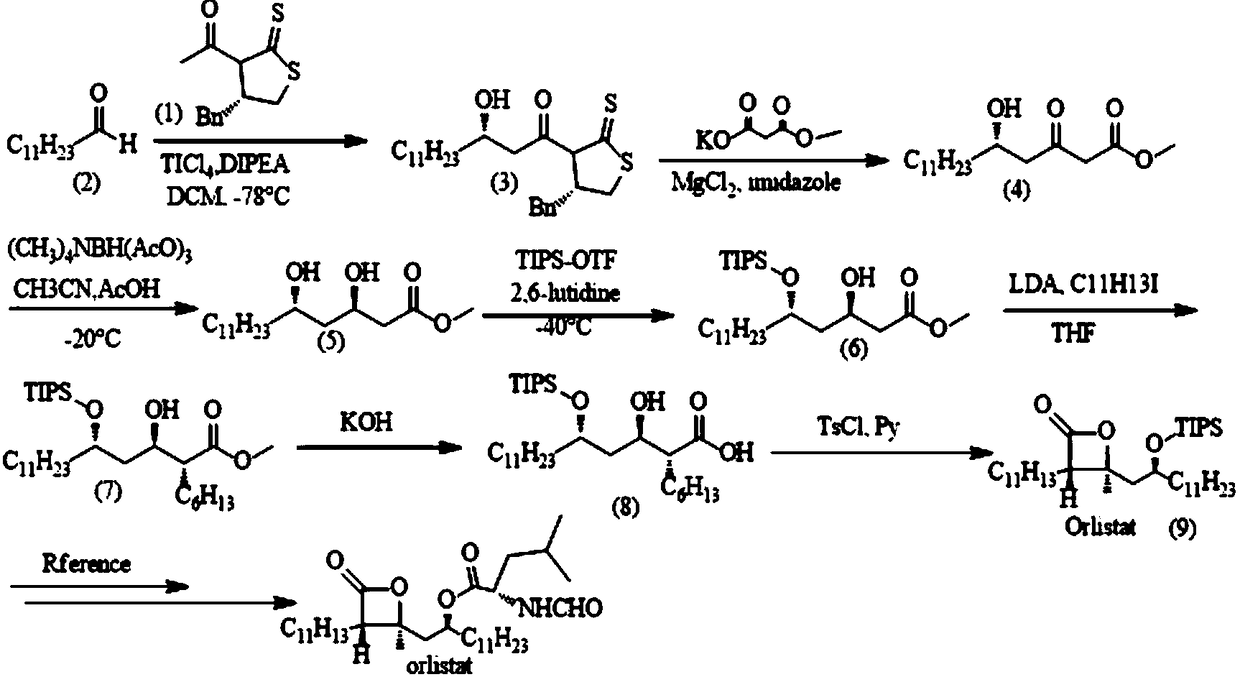
Synthesizing method of weight loss drug orlistat intermediate
ActiveCN108484536ANovel synthetic routeSimple and fast operationOrganic chemistryOrlistatWeight-loss drugs
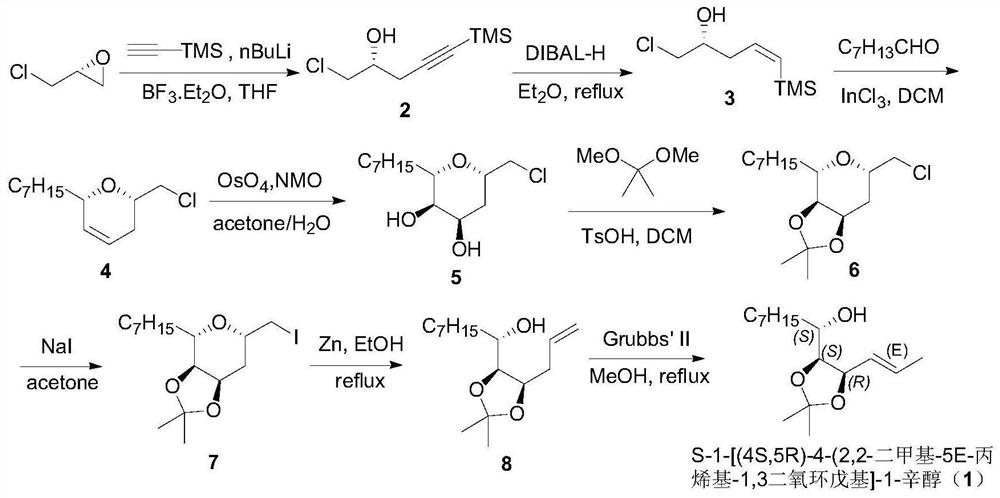
The invention discloses a method for synthesizing weight loss drug orlistat key intermediate. According to the method disclosed by the invention, n-dodecanal is utilized as a beginning raw material, orlistat intermediate compound (9) is prepared through multiple steps of reaction, and finally, orlistat can be prepared through two steps of known reaction of chiral hydroxyl deprotection reaction andmistsunobu reaction. A synthesizing strategy of the method disclosed by the invention is a typical linear strategy, a route design is simple and reasonable, an operation technology is convenient, reaction conditions are moderate, steps are few, a product yield is high, production cost is remarkably reduced, and the method is suitable for industrial preparation.
Owner:DONGGUAN UNIV OF TECH
Biosynthesis method of dapoxetine intermediate and dapoxetine intermediate
ActiveCN110078632ANovel synthetic routeLow costOrganic compound preparationCarbonyl compound preparationSynthesis methodsSubstitution reaction
The invention discloses a biosynthesis method of a dapoxetine intermediate and the dapoxetine intermediate. According to the method, a compound (2) and a compound (3) are used as initial materials andsubjected to a phase-transfer catalysis substitution reaction to prepare a compound (4), the compound (4) is subjected to a bioenzyme conversion reaction to prepare the dapoxetine intermediate compound (1) finally. The reaction formula of the compound is shown in the description. The synthesis method of the dapoxetine intermediate has the advantages that the method is novel in synthesis route, simple, feasible, lower in cost, high in synthesis yield and high in yield, the product has good purity and better quality, raw materials are cheap and easy to obtain and the method is suitable for industrial production and the like; meanwhile, the synthesized dapoxetine intermediate provides a novel intermediate raw material for preparation of dapoxetine.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
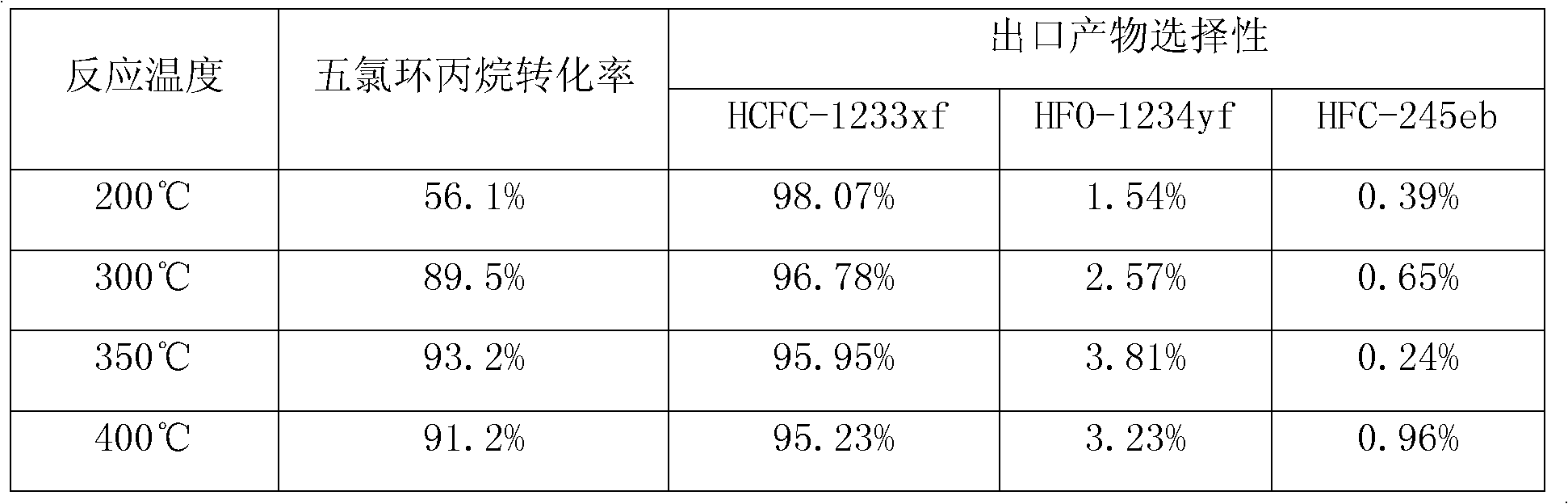
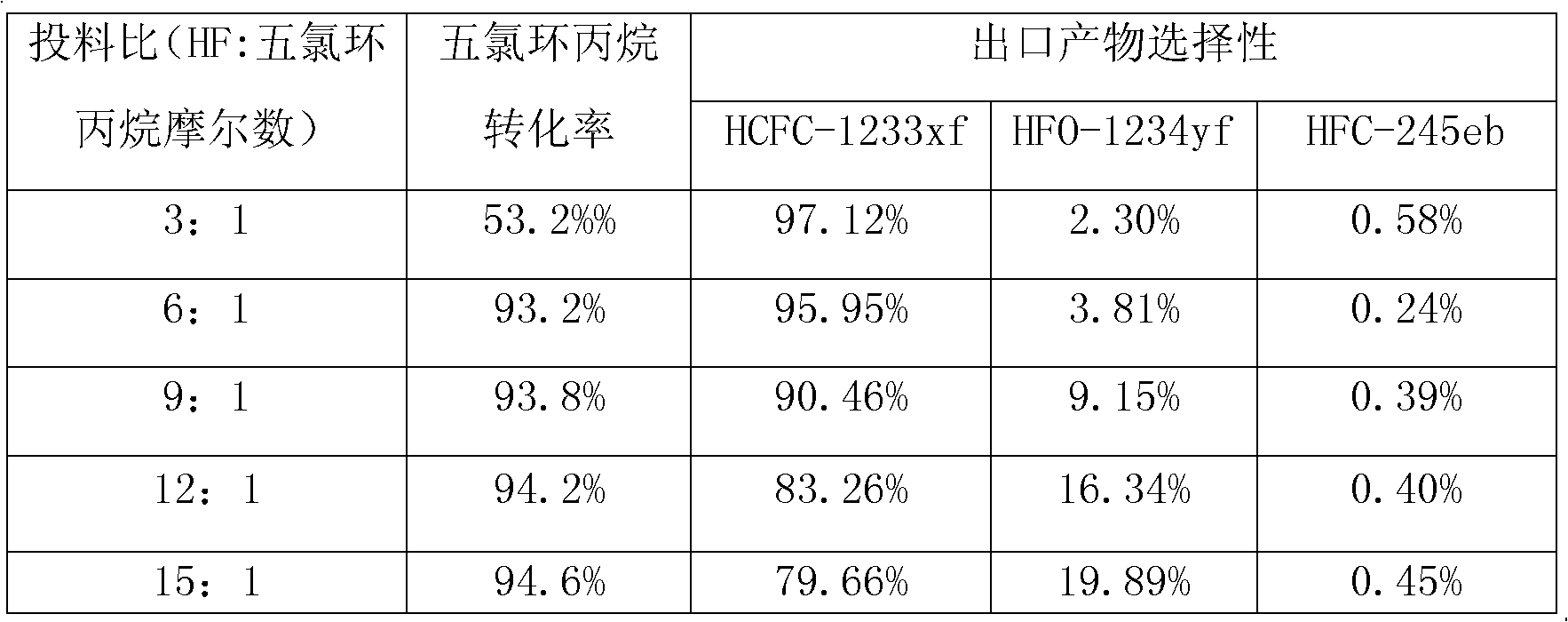
Pentachlorocyclopropane preparation method
ActiveCN102992947AHigh yieldNovel synthetic routePreparation by halogen additionCombinatorial chemistryRaw material
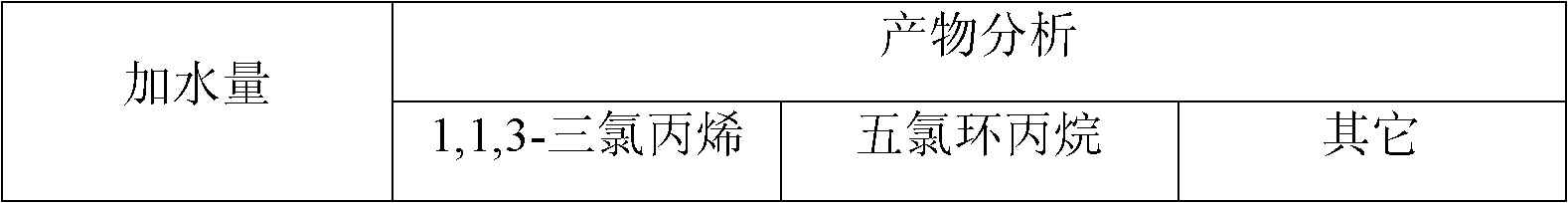
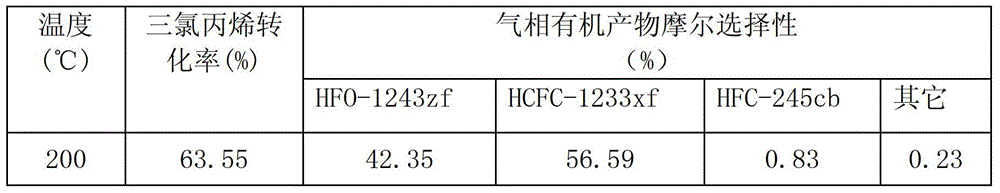
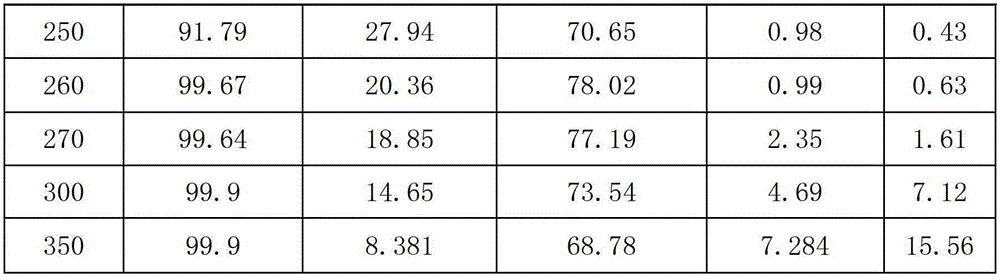
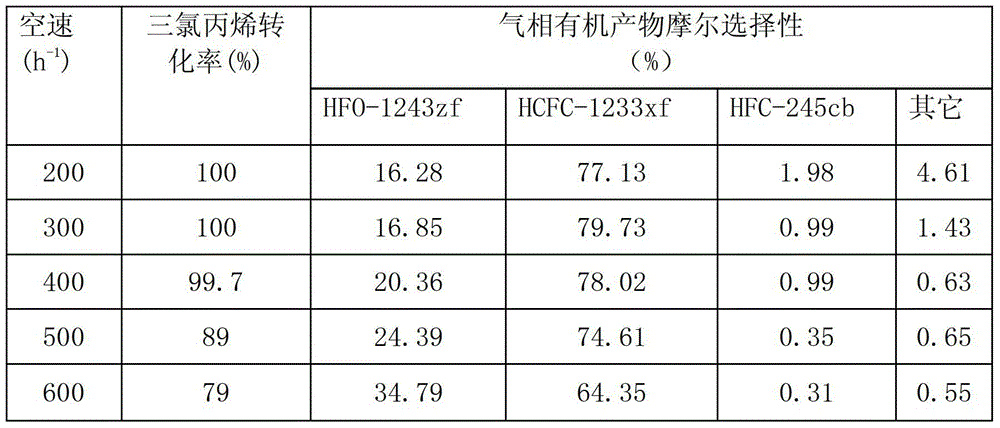
The invention provides a pentachlorocyclopropane preparation method. Pentachlorocyclopropane is generated through chlorinating 3,3,3-trichloropropylene which is a raw material by chlorine at 0-100DEG C. The pentachlorocyclopropane preparation method has the advantages of novel synthetic route, high product yield, simple operation, easy industrialized amplification and the like. The synthesized pentachlorocyclopropane can be used for synthesizing other fluorine-containing compounds.
Owner:SINOCHEM LANTIAN +1
Preparation method of photocurable hyperbranched polyurethane-epoxy acrylate
ActiveCN114149565ASimple methodNo "three wastes" producedChemical industryPolyurea/polyurethane coatingsPolymer scienceAcrylate ester
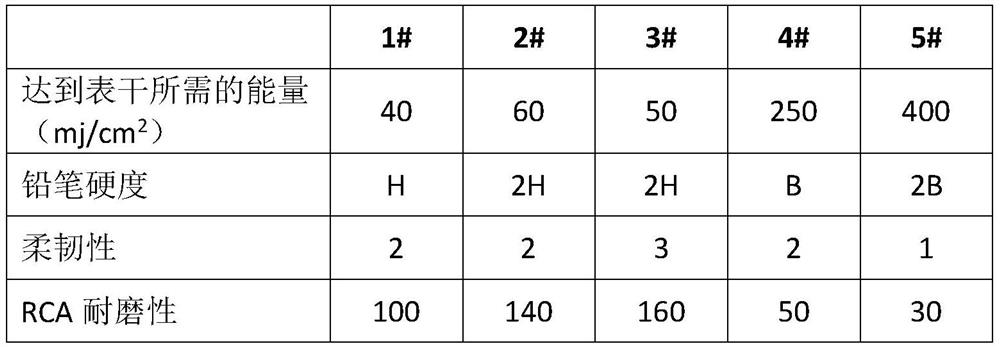
The invention discloses a preparation method of photocurable hyperbranched polyurethane-epoxy acrylate, which comprises the following steps: firstly synthesizing hyperbranched polyisocyanate, then reacting with glycidyl to obtain hyperbranched polyurethane-epoxy resin, and finally performing ring-opening reaction with acrylic acid or methacrylic acid to obtain the photocurable hyperbranched polyurethane-epoxy acrylate. And the photocurable hyperbranched polyurethane-epoxy acrylate is obtained. The method is simple and easy to implement and high in feasibility, the prepared photocurable hyperbranched polyurethane-epoxy acrylate is high in curing speed, and a cured film is high in hardness, good in flexibility and good in wear resistance.
Owner:江苏三木化工股份有限公司 +1
Florfenicol intermediate preparation method
InactiveCN113402475ANovel synthetic routeShorten the timeOrganic chemistry methodsPhenyl groupMethyl palmoxirate
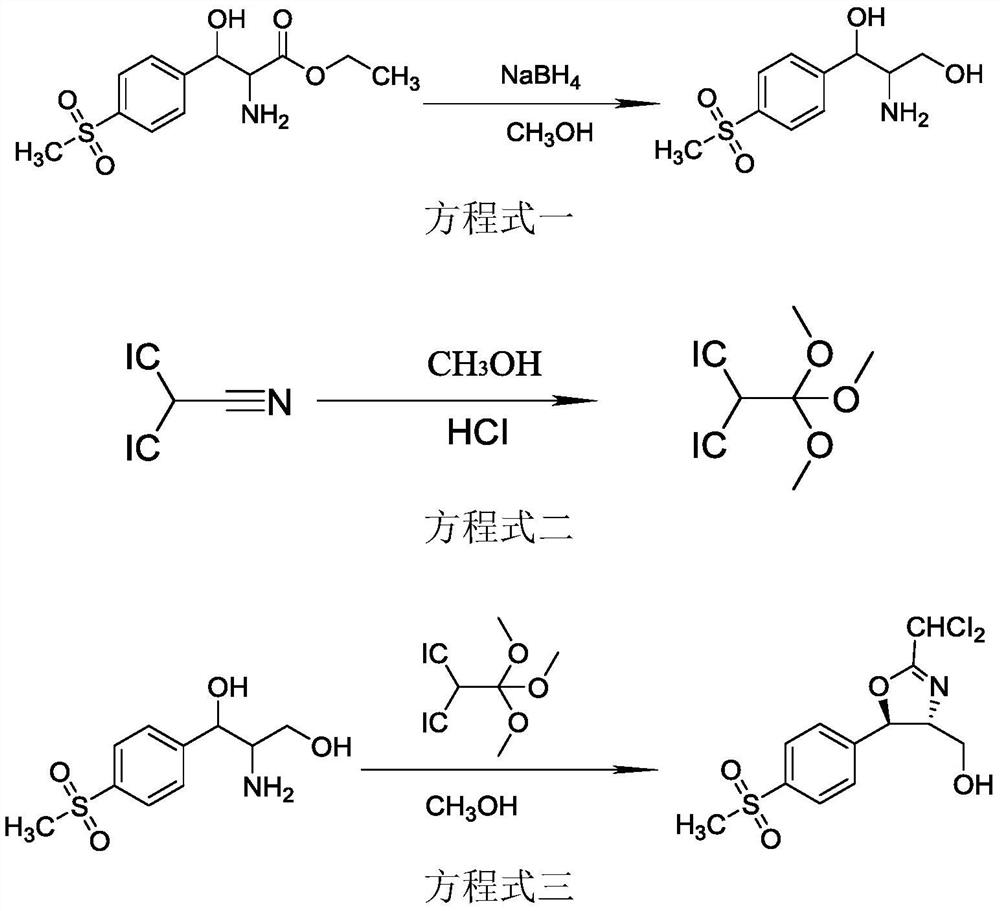
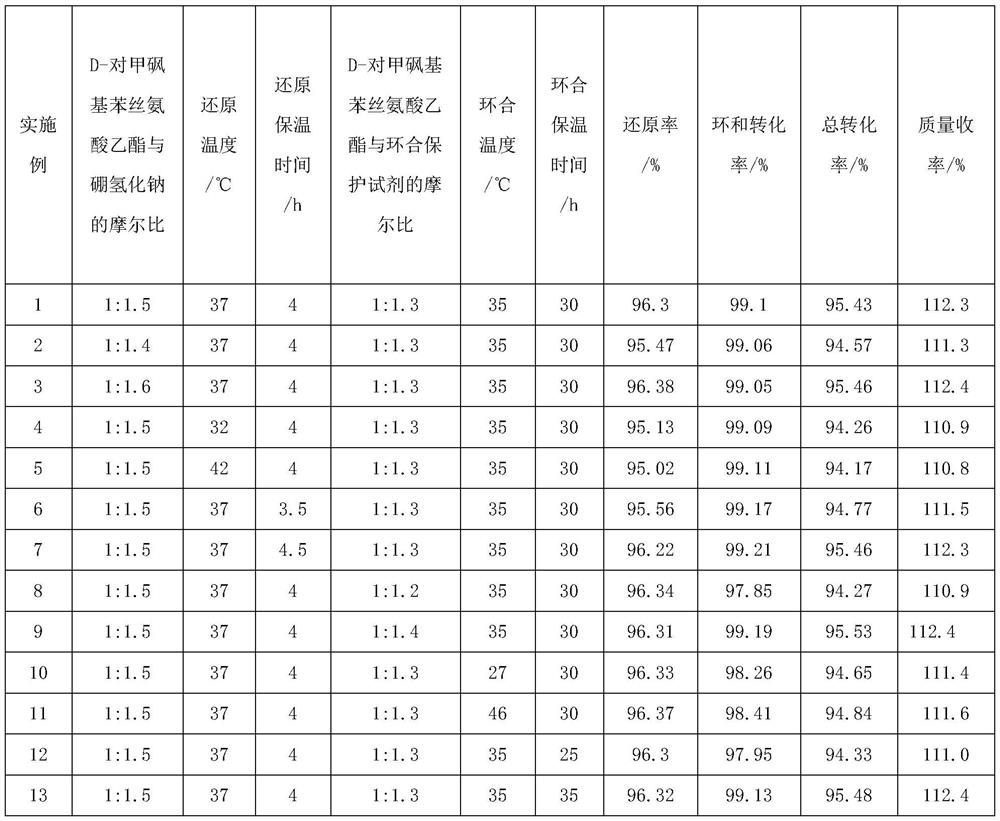
The invention discloses a florfenicol intermediate preparation method, which comprises: carrying out a reaction by using D-p-methylsulfonyl phenyl serine ethyl ester as a raw material, and reducing by using sodium borohydride as a reducing agent to obtain D-threon-2-amino-1-p-methylsulfonyl phenyl-1, 3-propylene glycol; preparing a cyclization protection reagent 1, 1-trimethyl dichloroorthoacetate from dichloroacetonitrile, methanol and hydrochloric acid; reacting D-threon-2-amino-1-p-methylsulfonyl phenyl-1, 3-propylene glycol with 1, 1-trimethyl dichloroorthoacetate to obtain the florfenicol intermediate D-threon-2-(dichloromethyl)-4, 5-dihydro-5-[4-(methylsulfonyl)-phenyl]-4-oxazole methanol. The method overcomes the defects of the prior art, shortens the reaction time, improves the yield, reduces the production cost, is simple to operate, and is suitable for industrial production.
Owner:SHANDONG GUOBANG PHARMA +1
A kind of synthetic method of hydroquinone
InactiveCN103420803BNovel synthetic routeThe process is simple and feasibleOrganic chemistryOrganic compound preparationQuinoneHydrogen
The invention discloses a hydroquinone synthetic method. The method comprises the steps that sodium phenate is reacted with ozone on the condition that catalyst hydroxyapatite exists, and benzene quinone is obtained after reaction liquid water steam is distilled; hydroquinone is obtained after hydrogen is added into the benzene quinone on the condition that catalyst raney nickel exists. The hydroquinone synthetic method is simple in synthetic route, novel, simple in technology, high in yield and purity of products, incapable of influencing the environment, and suitable for industrial production. Moreover, catalyst is cheap and can be obtained easily.
Owner:CHONGQING TECH & BUSINESS UNIV
2-chloro-3,3,3-trifluoropropylene preparation method
ActiveCN102992946ANovel synthetic routeRaw materials are easy to getPreparation by halogen replacementCyclopropaneEthyl Chloride
The invention provides a 2-chloro-3,3,3-trifluoropropylene preparation method. 2-chloro-3,3,3-trifluoropropylene is prepared through reacting halogenated cyclopropane which is a raw material and has a formula of C3HClXmYn with HF under the action of a fluorination catalyst, X and Y in the C3HClXmYn are independently selected from F, Cl, Br and I, m and n are integers in a range of 0-4, and the m+n value is 4. The method has the advantages of novel synthetic route, easily available raw material, high selectivity, high conversion rate and the like. The synthesized 2-chloro-3,3,3-trifluoropropylene can be used for preparing 2,3,3,3-tetrafluoropropylene.
Owner:SINOCHEM LANTIAN +1
Salts of dialkylphosphinic acid, preparation method and application
ActiveCN102718798BAvoid dangerAvoid it happening againGroup 5/15 element organic compoundsPhosphatePhosphoric acid
The invention discloses a preparation method and an application of salts of dialkylphosphinic acid, the salts of dialkylphosphinic acid has a structure shown in a formula (I). The preparation method of the salts of dialkylphosphinic acid comprises the following steps: hypophosphorous acid or hypophosphites are reacted with aldehyde in an aqueous solution to obtain di(alpha-hydroxy)alkyl hypophosphorous acid, hydroxy is reduced through HI / P to obtain an dialkylphosphinic acid solution, and the dialkylphosphinic acid solution and metal salt are reacted to obtain the corresponding salts of dialkylphosphinic acid. The preparation method of salts of dialkylphosphinic acid has the advantages of high yield, simple synthesis process and convenient operation, and substantially reduces production cost, simplifies production equipment and enhances the security of production process.
Owner:KINGFA SCI & TECH CO LTD +1
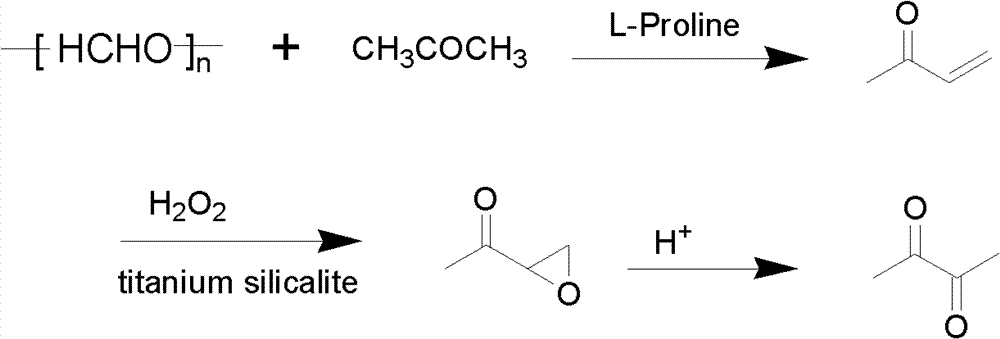
Method for preparing 2,3-butanedione from paraformaldehyde
InactiveCN101973860BReduce dosageImprove conversion ratePreparation from heterocyclic compoundsCatalytic oxidationKetone
The invention discloses a method for preparing 2,3-butanedione from paraformaldehyde. The method comprises the following steps of: preparing methyl vinyl ketone in one step from paraformaldehyde and acetone which serve as raw materials and L-proline serving as a catalyst through a condensation reaction of aldehydes and ketone and a dehydration reaction; and preparing 1,2-epoxy butanone from the methyl vinyl ketone serving as a raw material, hydrogen peroxide serving as an oxidant and a titanium and silicon molecular sieve serving as a catalyst by a catalytic oxidation method, and adding sodium hydrogensulfite for heating, and performing pinacol rearrangement to prepare 2,3-butanedione. Compared with the conventional method for preparing the 2,3-butanedione, the method has the characteristics of low cost, environmental-friendliness, high conversion rate of the paraformaldehyde and selectivity of the 2,3-butanedione and the like, and provides an environmental-friendly synthetic method for the industrial production of the 2,3-butanedione.
Owner:HUNAN NORMAL UNIVERSITY
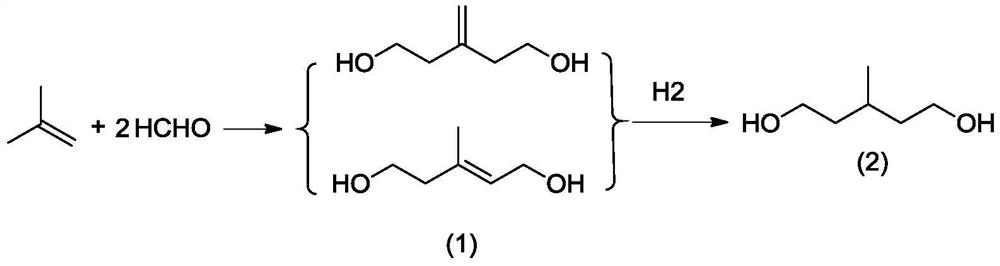
Preparation method of 3-methyl-1, 5-pentanediol
ActiveCN113880689ANovel synthetic routeHigh yieldOrganic compound preparationPreparation by hydrogenationPyridiniumPtru catalyst
The invention provides a preparation method of 3-methyl-1, 5-pentanediol, which comprises the following steps of: (1) carrying out a condensation reaction on isobutene and formaldehyde as raw materials under the action of an acid catalyst and an auxiliary agent to generate 3-methylene pentane-1, 5-diol and 3-methyl pentyl-2-ene-1, 5-pentanediol; and (2) carrying out a hydrogenation reaction by taking the 3-methylene pentane-1, 5-diol and the 3-methyl pentyl-2-ene-1, 5-pentanediol as raw materials to obtain 3-methyl-1, 5-pentanediol. A sulfoacid pyridinium salt is used as a catalyst and phosphate is used as an auxiliary agent in the condensation reaction of isobutene and formaldehyde, so that the product yield can be obviously improved. The method has the advantages of short process steps, high yield and low cost, and is suitable for industrial amplification.
Owner:WANHUA CHEM GRP CO LTD
A kind of biosynthesis method of dapoxetine intermediate and intermediate thereof
ActiveCN110078632BNovel synthetic routeLow costOrganic compound preparationCarbonyl compound preparationCombinatorial chemistryDapoxetine-N-oxide
The invention discloses a biosynthesis method of a dapoxetine intermediate and an intermediate thereof. The method uses compound (2) and compound (3) as starting materials, and prepares compound (4) through a phase transfer catalytic substitution reaction , the compound (4) finally obtains the dapoxetine intermediate compound (1) through a biological enzyme conversion reaction, and its reaction formula is as follows: The biosynthesis method of the dapoxetine intermediate of the present invention has a novel synthetic route, Simple and easy to implement, low cost, high synthetic yield, high yield, good product purity, good product quality, cheap and easy-to-get raw materials, and suitable for industrial production. The preparation of poxetine provides a new intermediate raw material.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
A kind of method for preparing octafluoro-2-butene
ActiveCN110396037BNovel synthetic routeRaw materials are easy to getHalogenated hydrocarbon preparationButenePtru catalyst
The invention discloses a method for preparing octafluoro-2-butene, comprising Cu + Under the action of a procatalyst and a cocatalyst including an organic amine, 2,2‑dichloro1,1,1,2‑tetrafluoroethane reacts with copper to give 1,1,1,2,3,4,4,4 ‑Octafluoro‑2‑butene. The preparation method provided by the invention has the advantages of novel synthetic route, readily available raw materials, low cost and suitable for industrial production.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
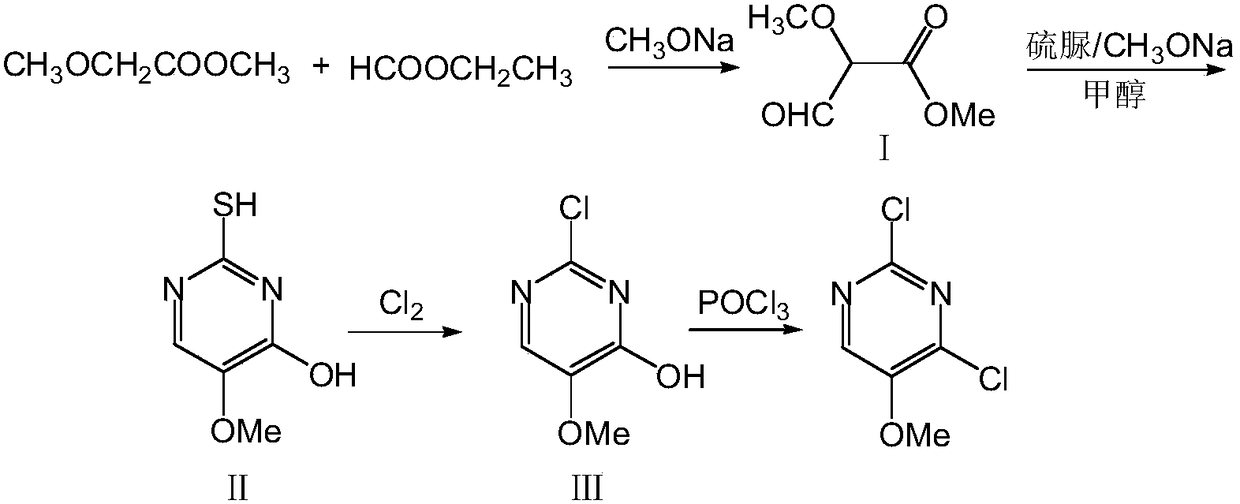
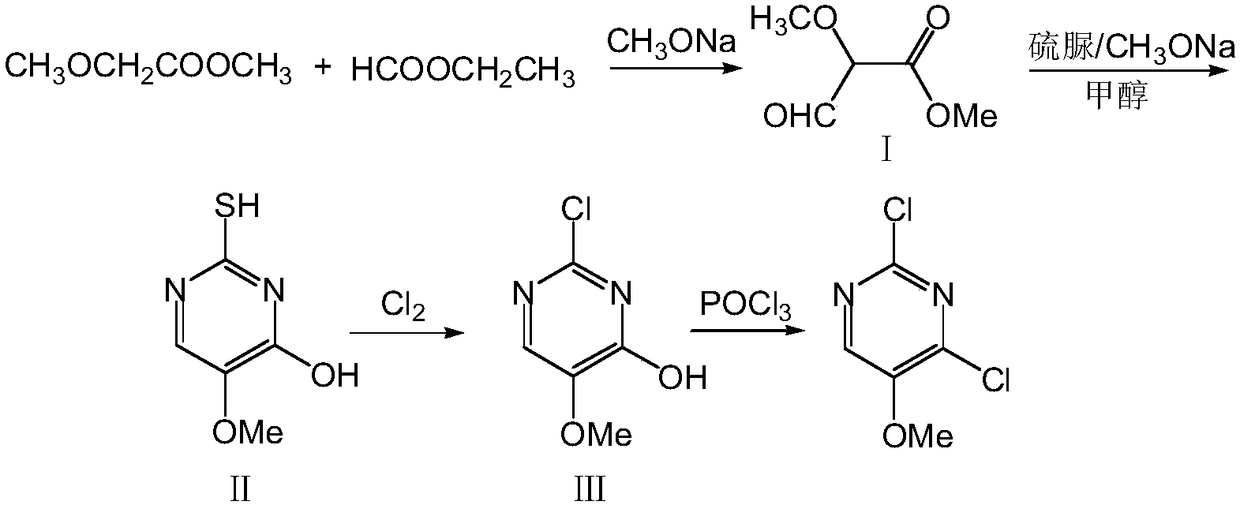
A kind of preparation method of 2,4-dichloro-5-methoxypyrimidine
ActiveCN106187914BReduce dosageRaw materials are easy to getOrganic chemistryThiolMethyl methoxyacetate
Owner:CHAMBROAD CHEM IND RES INST CO LTD
Method for synthesizing styrallyl acetate from acetophenone
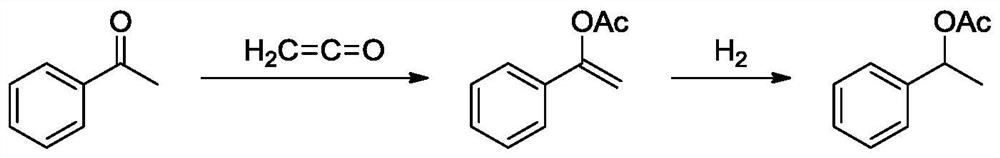
ActiveCN112479879ALow priceNovel synthetic routeOrganic compound preparationPreparation from ketenes/polyketenesOrganic acidEthenone
The invention provides a method for synthesizing styrallyl acetate from acetophenone, which comprises the following steps: reacting acetophenone with ketene under the catalysis of organic acid to obtain styrene acetate, and hydrogenating styrene acetate to obtain styrallyl acetate. The method is novel in synthetic route, the raw materials acetophenone and ketene are cheap and easy to obtain, the synthetic route is short, the yield is high, no equivalent acetic acid byproduct is produced, and the method has an obvious cost advantage.
Owner:WANHUA CHEM GRP CO LTD
Eriocalyxin A and Eriocalyxin B and preparation method thereof
PendingCN114805270ANovel synthetic routeOrganic active ingredientsOrganic chemistry methodsChemical compoundOrganic synthesis
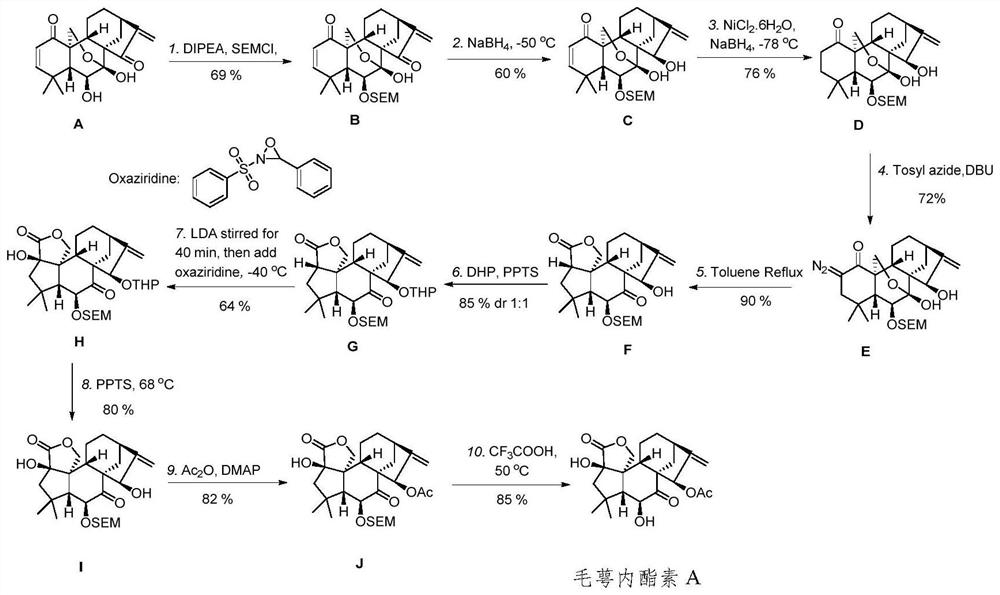
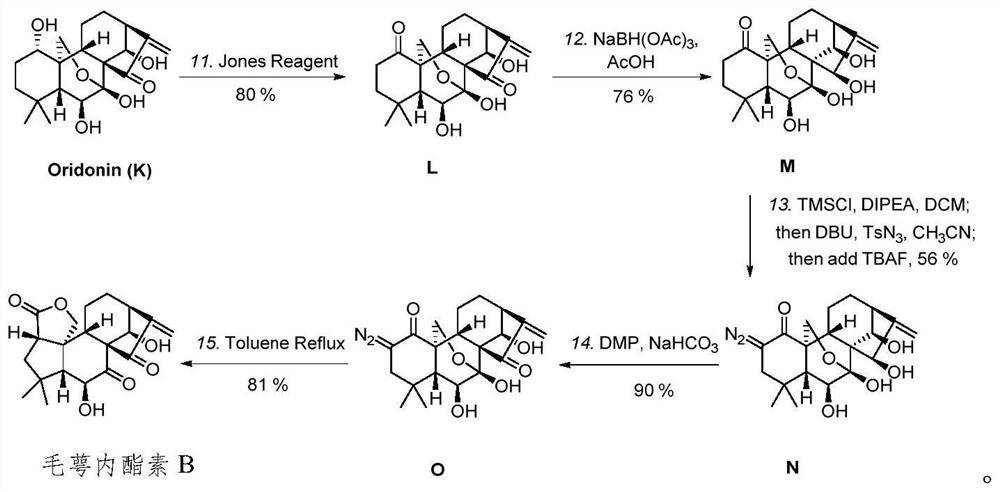
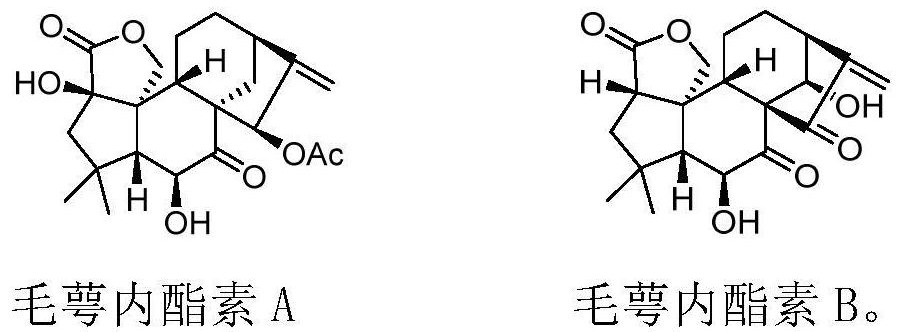
The invention discloses eriocalyxin A and B and a preparation method thereof, and belongs to the technical field of organic synthesis of natural products and the technical field of medicines. The invention provides a novel pentacyclic enantiomer-kaurane diterpenoid compound (eriocalyxin A and eriocalyxin B) and a preparation method of the novel pentacyclic enantiomer-kaurane diterpenoid compound (eriocalyxin A and eriocalyxin B). The compound is synthesized for the first time, the synthesis route is novel, the synthesis method can provide an effective means for structure optimization based on oridonin, and the compound has important research value and application prospects.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
A kind of asymmetric cyclobutane derivative and its preparation method and application
ActiveCN105294639BGood immunosuppressive activityBiologically activeOrganic active ingredientsOrganic chemistryChemical synthesisSide chain
The invention discloses asymmetric cyclobutane derivatives (synthetic derivatives G of rabdosia scoparia lactones), which are named as scopariusicide derivatives, a pharmaceutical composition taking the asymmetric cyclobutane derivatives as the active component, a preparation method of asymmetric cyclobutane derivatives, and an application of asymmetric cyclobutane derivatives in preparation of immuno-suppressive drugs. According to the preparation, scopariusicides, which is derived from pharmaceutical plants, is taken as the template; a precursor compound ent-clerodane diterpene A is taken as the initial raw material, a series of artificial analogues are obtained through six steps of chemical synthesis; the basic structure of the derivatives has a characteristic that ent-clerodane hetero-diterpene has an asymmetric cyclobutane segment, the side chain and aromatic ring of the derivatives may have different substituents, and thus there are many kinds of derivatives. The compounds are synthesized for the first time, the synthesis route is novel, and the derivatives have potential bioactivity, can be used as the lead compounds of immuno-suppressive drugs, and have a very important research value and application prospect.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
A method for preparing 2-chloro-3,3,3-trifluoropropene
ActiveCN104163751BThe reaction steps are simpleAvoid cokingPreparation by halogen replacementEthyl ChlorideMedicinal chemistry
The invention discloses a method for preparing 2-chloro-3,3,3-trifluoropropene from trichloropropene. First, 1,1,3-trichloropropene and / or 3,3,3-trichloropropene, HCl and O 2 The gas phase mixture of the mixture is used as a raw material, reacted under the action of an oxychlorination catalyst, and then passed through anhydrous HF under the action of a fluorination catalyst for further reaction to obtain 2-chloro-3,3,3-trifluoropropene. The 2-chloro-3,3,3-trifluoropropene prepared by the invention can be used to synthesize 2,3,3,3-tetrafluoropropene.
Owner:SINOCHEM LANTIAN +1
A kind of preparation method of pharmaceutical intermediate
ActiveCN109776479BEasy to operateRaw materials are cheap and easy to getOrganic chemistryIsomerizationDouble bond
Owner:SHAOXING UNIVERSITY
A kind of cyclic aminopyrimidine derivative and its activity and application of inhibiting kinase
ActiveCN111269245BGood clinical valueNovel synthetic routeOrganic chemistryAntineoplastic agentsPharmaceutical medicinePerylene derivatives
Owner:HANGZHOU BIO CREATIVITY PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com