Cyclic aminopyrimidine derivatives as well as kinase inhibition activity and application thereof
A technology of aminopyrimidine and derivatives, applied in the field of medicine, achieves the effects of good production feasibility, novel synthesis route and huge clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of compound T-1.
[0068]
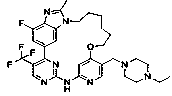
[0069] Compound T-1: ( E )-4 4 -((4-Ethylpiperazin-1-yl)methyl)-1 4 ,2 5 -Difluoro-1 2 -Methyl-1 1 H -5-Oxa-3-aza-1(6,1)-benzo[ d ]imidazole-2(4,2)-pyrimidine-4(1,3)-benzocycloundec-8-ene was synthesized according to the general procedure shown below:
[0070]
[0071]
[0072] Scheme 1 T-1 synthetic route.
[0073] Step 1: 4-(1-(3-buten-1-yl)-4-fluoro-2-methyl-1 H -Benzo[ d ]imidazol-6-yl)-N-(3-(3-butene-1-oxyl)-4-((4-ethylpiperazin-1-yl)methyl)phenyl)-5-fluoro Synthesis of pyrimidin-2-amine (1).
[0074] Intermediate Z-1 (0.27 g, 0.8 mmol), W1-1 (0.29 g, 1.0 mmol), cesium carbonate (0.52 g, 1.6 mmol), 4,5-bisdiphenylphosphine-9,9-dimethyl Xanthoxanthene (Xantphos, 0.093 g, 0.16 mmol) was dissolved in 10 mL of 1,4-dioxane, nitrogen was replaced three times, and Pd was added 2 (dba) 3 , heated to 90°C for 3 hours, poured the reaction solution into 30 mL water and extracted with ethyl acetate (20 mL x 2), com...
Embodiment 3
[0119] Synthesis of Compound T-3.
[0120]
[0121] Compound T-3: ( E )-4 5 -((4-Ethylpiperazin-1-yl)methyl)-1 4 ,2 5 -difluoro-1 2 -Methyl-1 1 H -5-oxa-3-aza-1 (6, 1)-benzo[d]imidazole-2 (4, 2)-pyrimidine-4 (2, 4)-pyridocycloundecane-8- Alkenes are shown as follows:
[0122]
[0123] .
[0124] Referring to the synthesis method of Example 1, a yellow solid T-3 was obtained, MS (m / z): 561.3 [M+H] + .
[0125] Synthetic route of side chain W3-1:
[0126]
[0127]
[0128] Step 1: Synthesis of (4,6-dichloropyridin-3-yl)(4-ethylpiperazin-1-yl)methanone (22).
[0129] 4,6-Dichloronicotinic acid (21, 3.8 g, 20 mmol) was dissolved in 100 mL toluene, added 3 mL thionyl chloride, reacted at 100°C for 5 hours, spin-dried the solvent, dissolved in DCM, -10°C Add ethylpiperazine (13, 2.3 g, 20 mmol) and triethylamine (2.0 g, 20 mmol) dropwise to the DCM reaction liquid under the conditions, react for 1 hour, spin to dry the solvent, dissolve in 50 mL EA, and was...
Embodiment 4
[0141] Synthesis of T-27.
[0142]
[0143] Compound T-27: (1 3 Z ,1 4 E ,10 E )--1 7 ,2 5 -difluoro-1 2 -Methyl-4 4 -((4-Ethylpiperazin-1-yl)-methyl-)-1 2 H -5-oxa-3-aza-1(5,3)-indazole-2(4,2)-pyrimidine-4(1,3)-benzocyclododec-10-ene is as follows The general operation shown is synthetic:
[0144]
[0145]
[0146]
[0147] Synthetic route of Scheme 8 T-27.
[0148] step 1:( E )-N-(3-((6-bromo-5-hexen-1-yl)oxy) 4-((4-ethylpiperazin-1-yl)methyl)phenyl)-5-fluoro -4-(7-fluoro-3-iodo-2-methyl-2 H -Synthesis of indazol-5-yl)pyrimidin-2-amine (27).
[0149] Intermediate Z-2 (0.32 g, 0.8 mmol), W1-2 (0.4 g, 1.0 mmol), cesium carbonate (0.52 g, 1.6 mmol), Xantphos (0.093 g, 0.16 mmol) were dissolved in 10 mL of 1,4- In dioxane, replace nitrogen three times, add Pd 2 (dba) 3 , heated to 90°C and reacted for 3 hours, poured the reaction solution into 30 mL water and extracted with ethyl acetate (20 mLx 2), combined the organic phases, washed with saturated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com