A kind of preparation method of 2,4-dichloro-5-methoxypyrimidine
A technology of methoxypyrimidine and mercapto, which is applied in the field of preparation of 2,4-dichloro-5-methoxypyrimidine, can solve the problems of low yield and large amount of chlorinating agent, and achieve high yield and chlorine The effect of low dosage of agent and novel synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
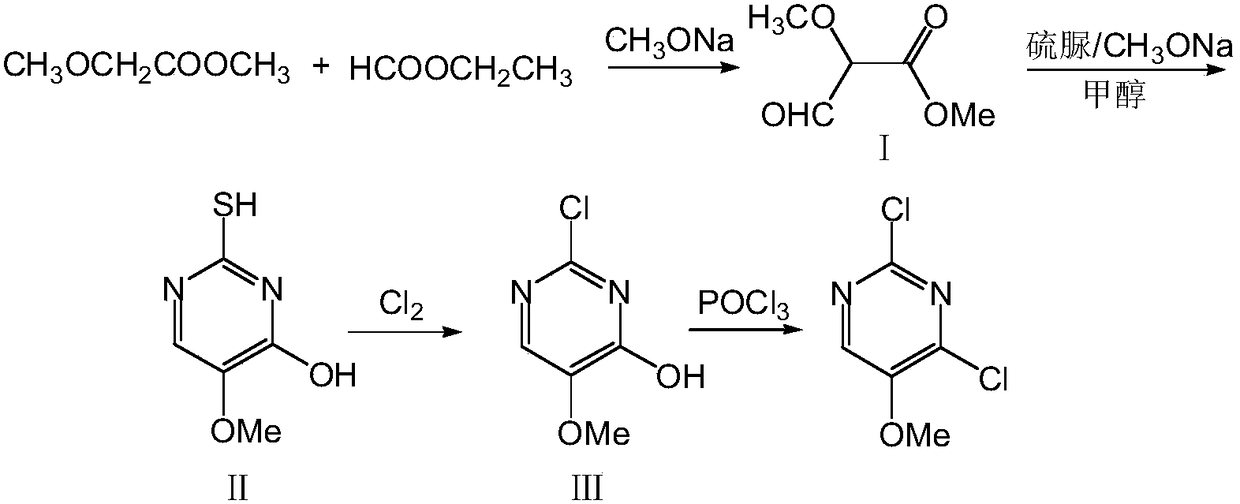
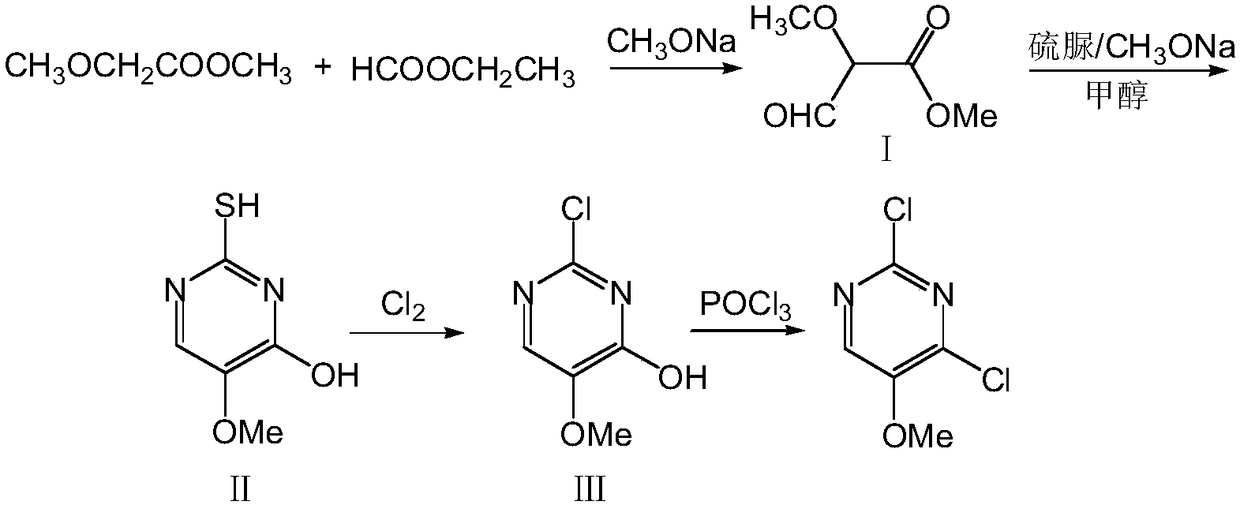
[0020] A) To prepare 2-mercapto-4-hydroxy-5-methoxypyrimidine, add 185 g (2.5 mol) of ethyl formate into the device, add 91.8 g (1.7 mol) of sodium methoxide solid under stirring, and add dropwise to the system below 20°C 104g (1mol) of methyl methoxyacetate, the dropwise addition was completed and the temperature was kept at 20°C for 300min to obtain compound I; then, 340ml of methanol was added to compound I and heated to 65°C and stirred until compound I was uniformly dispersed, and 114g (1.5mol) of thiourea was added. ), reflux for 260min, concentrate to 1 / 4 volume, add 330ml of water to dissolve, cool to 10°C, adjust the pH value to 2 with hydrochloric acid, filter, wash the filter cake twice with 100ml of water, dry at 100°C for 8h to obtain 145.3g of compound Ⅱ, the yield was 92%, and the HPLC purity was 99.7%;
[0021] B) To prepare 2,4-dichloro-5-methoxypyrimidine, in a 500ml four-necked flask equipped with a thermometer, reflux condenser, ventilation device and mecha...
Embodiment 2
[0023] A) Prepare 2-mercapto-4-hydroxyl-5-methoxypyrimidine, using the same method as in Example 1 to obtain compound I; then add 340ml methanol to compound I and heat to 65°C and stir until compound I is uniformly dispersed, add sulfur Urea 152g (2mol), reflux for 240min, concentrate to 1 / 4 volume, add 330ml water to dissolve, cool to 10°C, adjust pH value to 3 with acetic acid, filter, wash filter cake twice with 100ml water, dry at 100°C for 8h 150.1 g of compound II was obtained with a yield of 95% and a purity of 99.6% by HPLC;
[0024] B) To prepare 2,4-dichloro-5-methoxypyrimidine, in a 500ml four-necked flask equipped with a thermometer, reflux condenser, ventilation device and mechanical stirring, add 250ml dichloropyrimidine to compound II 79g (0.5mol) Ethane was passed through with chlorine gas at a rate of 3 g / min, and reacted at 20° C. for 400 min to obtain compound III. Then, 101 g (0.5 mol) of triethylamine and 84.4 g (0.55 mol) of phosphorus oxychloride were s...
Embodiment 3
[0026] A) Prepare 2-mercapto-4-hydroxyl-5-methoxypyrimidine, using the same method as in Example 1 to obtain compound I; then add 340ml methanol to compound I and heat to 65°C and stir until compound I is uniformly dispersed, add sulfur Urea 152g (3mol), reflux for 180min, concentrate to 1 / 4 volume, add 330ml water to dissolve, cool to 10°C, adjust pH value to 5 with sulfuric acid, filter, wash filter cake twice with 100ml water, dry at 100°C for 8h 144.25 g of compound II was obtained with a yield of 91.3% and a purity of 99.2% by HPLC;
[0027] B) To prepare 2,4-dichloro-5-methoxypyrimidine, in a 500ml four-necked flask equipped with a thermometer, reflux condenser, ventilation device and mechanical stirring, add 250ml dichloropyrimidine to compound II 79g (0.5mol) Ethane was passed through with chlorine gas at a rate of 1 g / min, and reacted at 30° C. for 420 min to obtain compound III. Then put 79g (1mol) of pyridine and 115.15g (0.75mol) of phosphorus oxychloride into the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com