Salts of dialkylphosphinic acid, preparation method and application
A technology of dialkyl phosphinate and dialkyl phosphinic acid, applied in the field of dialkyl phosphinate, can solve the problem of easy quenching and inactivation reaction yield of free radicals, environmental pollution by waste, and large input of raw materials. and other problems, to achieve the effect of novel synthetic route, high purity, and avoidance of danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
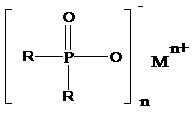
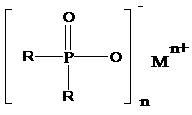
[0041] The present invention discloses a preparation method of dialkyl phosphinate represented by formula (I), comprising the following steps:
[0042]
[0043] (I);
[0044] Wherein, R is an alkyl group with 1 to 12 carbon atoms; n=2 or 3; M is a metal ion;
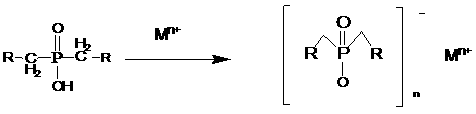
[0045] (1) Preparation of dihydroxyalkyl hypophosphorous acid: reaction of hypophosphorous acid and aldehyde in water to obtain dihydroxyalkyl hypophosphorous acid;
[0046] (2) Preparation of dialkylphosphinic acid: the hydroxyl group of dihydroxyalkylphosphinic acid is reduced by reducing agent to obtain dialkylphosphinic acid solution;
[0047] (3) Preparation of dialkylphosphinic acid salt: react the dialkylphosphinic acid solution obtained in step (2) with an aqueous metal salt solution to obtain a solid, which is cooled, filtered, washed with water, and dried to obtain a dialkylphosphinic acid solution. Phosphonates.
[0048] Wherein, the preparation of step (1) dihydroxyalkyl hypophosphorous acid can also ob...
Embodiment 1
[0053] (1) Preparation of bishydroxymethylphosphinic acid
[0054] In a 500mL four-necked flask equipped with a stirrer and a reflux condenser, add 159g (1.5mol) sodium hypophosphite monohydrate, 100mL deionized water, 135mL (1.55mol) 36% hydrochloric acid and 93g (3.1mol) Paraformaldehyde was stirred and reacted at 60°C for 10 hours to obtain 487g of bismethylolphosphinic acid aqueous solution, which contained 36.4% of bismethylolphosphinic acid (M=126), and the yield was 93%.
[0055] After purification, the product is analyzed by infrared spectrum: there are absorptions at υ(cm- 1 ) = 475, 769, 856, 1035, 1091, 1151, 1310, 1422, 1635, 2900 and 3406.
[0056] (2) Preparation of dimethylphosphinic acid
[0057] Take 200ml of bishydroxymethylphosphinic acid (0.578mol) aqueous solution, concentrate under reduced pressure to 100ml, add 50ml of 57% hydroiodic acid (d=1.7), 60g of red phosphorus, and reflux at 126°C for 15 hours. Cool, extract 3 times with 50ml of chloroform, co...
Embodiment 2
[0062] (1) Preparation of bishydroxymethylphosphinic acid
[0063] In a 500mL four-necked flask equipped with a stirrer and a reflux condenser, 198g (1.5mol) of 50% hypophosphorous acid, 120mL deionized water and 86g (3.1mol) of paraformaldehyde were added, and the reaction was stirred at 60°C for 10h to obtain 400 g of bismethylolphosphinic acid aqueous solution contained 42.5% of bismethylolphosphinic acid (M=126) according to liquid chromatography analysis, and the yield was 90%.
[0064] Infrared spectrum (IR) after purification of the product: there are absorptions at υ(cm-1) = 475, 769, 856, 1035, 1091, 1151, 1310, 1422, 1635, 2900, 3406.
[0065] (2) Preparation of dimethylphosphinic acid
[0066] Take 200ml of bishydroxymethylphosphinic acid (0.675mol) aqueous solution, concentrate under reduced pressure to 100ml, add 60ml of 57% hydroiodic acid (d=1.7), 70g of red phosphorus, and reflux at 126°C for 15 hours. Cooled, extracted 3 times with 50ml of chloroform, combin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com