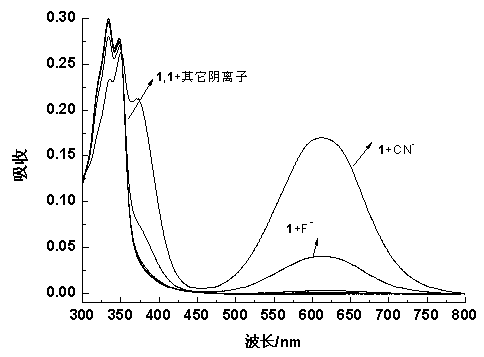
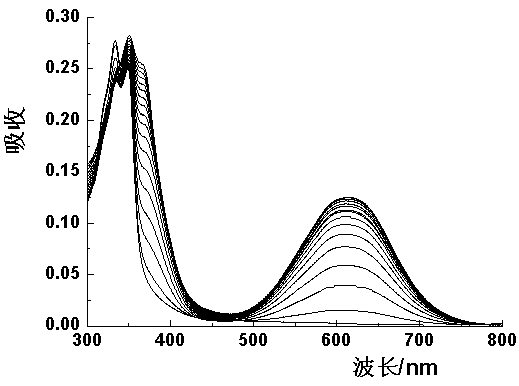
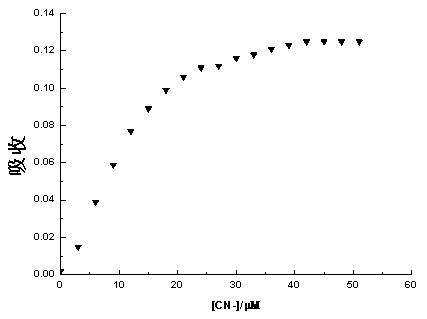
4-aroyl-1,8-naphthalimide compound and preparation method and use thereof
A technology of naphthalimide and compound, which is applied in the field of 4-aroyl-1,8-naphthoimide compound and its preparation, can solve the problems of low yield, many side reactions, harsh reaction conditions, etc., and achieve The effect of high yield, low production cost, and easy control of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A kind of 4-aryl acetonitrile-1,8-naphthalimide compound, its structural formula is as follows:
[0044]
[0045] where R 1 It is a C4 linear alkyl group;
[0046] R 2 For phenyl.
[0047] The preparation method of above-mentioned a kind of 4-aryl acetonitrile-1,8-naphthalimide compound, the reaction formula of its synthetic process is as follows:
[0048]
[0049] The preparation method of above-mentioned a kind of 4-aryl acetonitrile-1,8-naphthalimide compound, concrete steps are as follows:
[0050] In a 50ml three-neck flask, add 20ml of anhydrous toluene and phenylacetonitrile (0.40g, 3.42mmol), 0.20g of NaH solid, replace with nitrogen three times, stir magnetically for a period of time, and start adding N-butyl-4-bromo -1,8 Naphthalimide (1.0g, 3.02mmol) and 10ml of anhydrous toluene solution were added dropwise for about 30 minutes, after the drop was completed, stirred at room temperature for a period of time, followed by TLC spotting until the reaction...
Embodiment 2
[0058] A 4-arylacetonitrile-1,8-naphthalimide compound, its structural formula is as follows:
[0059]
[0060] where R 1 is n-butyl;
[0061] R 2 For 4-fluorophenyl.
[0062] The preparation method of above-mentioned a kind of 4-aryl acetonitrile-1,8-naphthalimide compound, the reaction formula of its synthetic process is as follows:
[0063]
[0064] The preparation method of above-mentioned a kind of 4-aryl acetonitrile-1,8-naphthalimide compound, concrete steps are as follows:
[0065] In a 100ml three-neck flask, add 25ml of anhydrous toluene and p-fluorophenylacetonitrile (0.50g, 3.70mmol), 0.25g of NaH solid, nitrogen replacement three times, after stirring at room temperature for a period of time, start to drop N-butyl- 4-Bromo-1,8 naphthalimide (1.10g, 3.32mmol) and 20ml of anhydrous toluene solution, after the dripping, began to heat for a period of time, TLC plate traced until the reaction was complete; Add 10% dilute hydrochloric acid solution, adjust th...
Embodiment 3
[0074] A kind of 4-aryl acetonitrile-1,8-naphthalimide compound, its structural formula is as follows:
[0075]
[0076] where R 1 is n-butyl;
[0077] R 2 Indolyl protected by BOC.
[0078] The preparation method of above-mentioned a kind of 4-aryl acetonitrile-1,8-naphthalimide compound, the reaction formula of its synthetic process is as follows:
[0079]
[0080] The preparation method of above-mentioned a kind of 4-aryl acetonitrile-1,8-naphthalimide compound, concrete steps are as follows:
[0081] Add 10ml of anhydrous toluene and sodium hydrogen (0.36g, 9.0mmol) to a 50ml three-necked flask at room temperature, and add indole-3-acetonitrile-N-methyl tert-butyl ester (0.29g, 1.13mmol) under nitrogen protection, 0.5 After 4h, 4-bromo-1,8-naphthoimide (0.25g, 0.76mmol) was added, and after 2h, the plate was followed to track the end of the reaction. After quenching with saturated brine, 50 ml of dilute hydrochloric acid was added to wash until acidic. The solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com