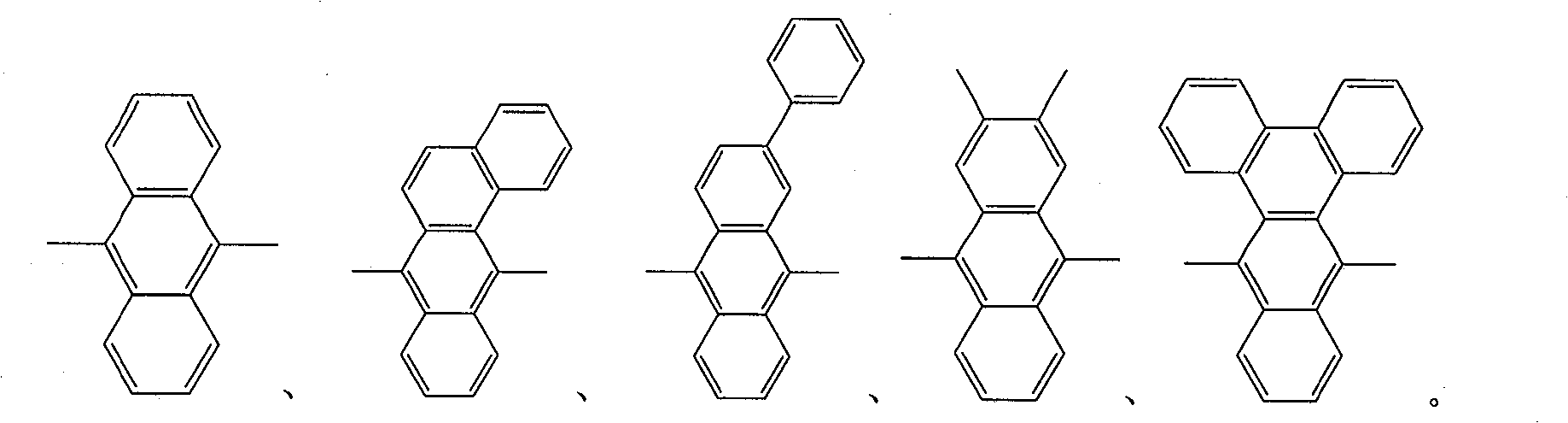
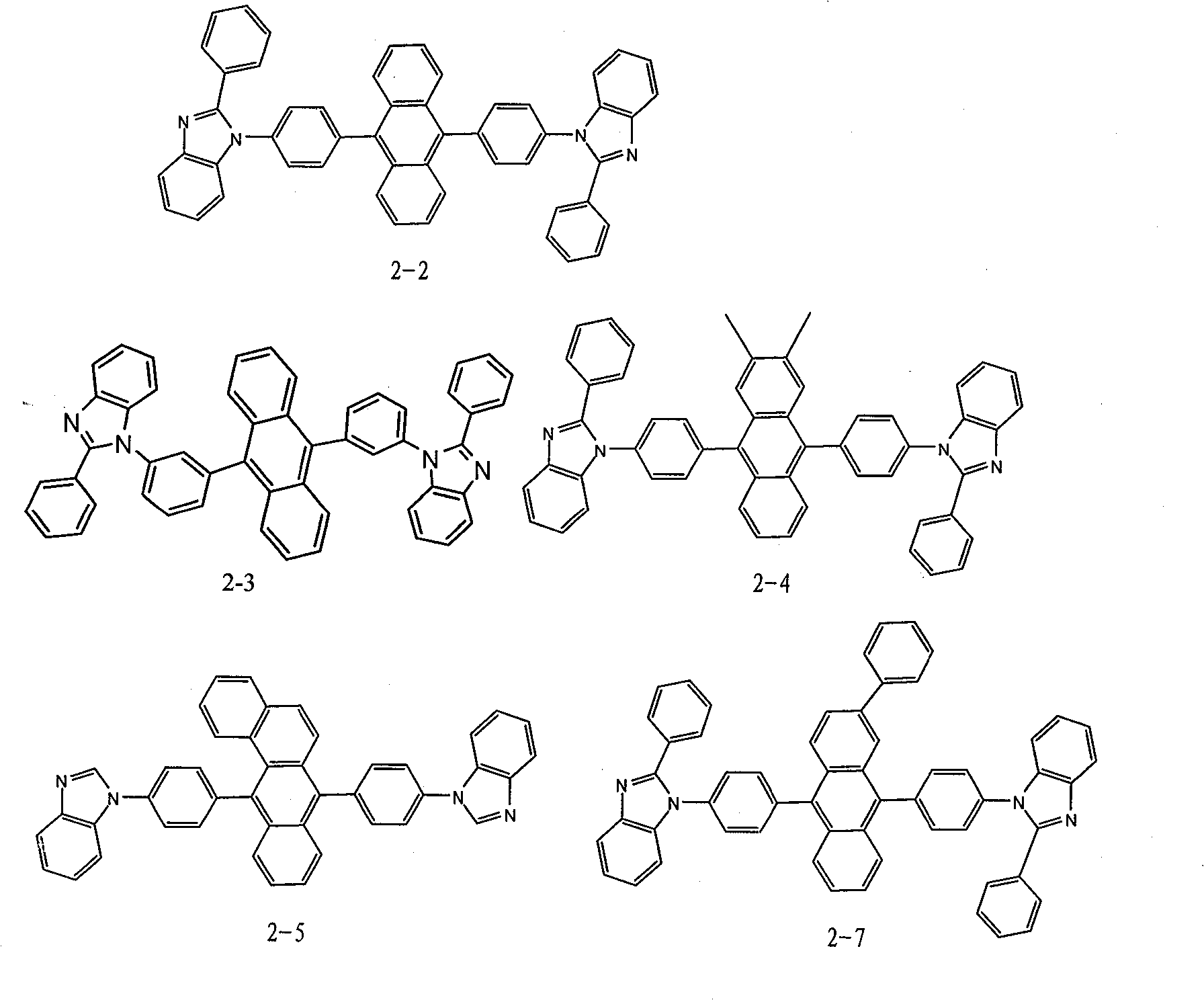
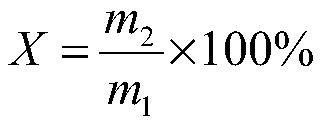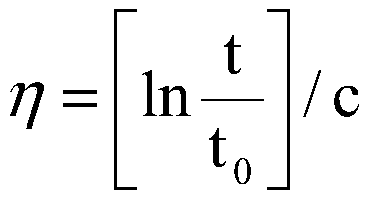Patents
Literature
313 results about "Phenyl-naphthylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
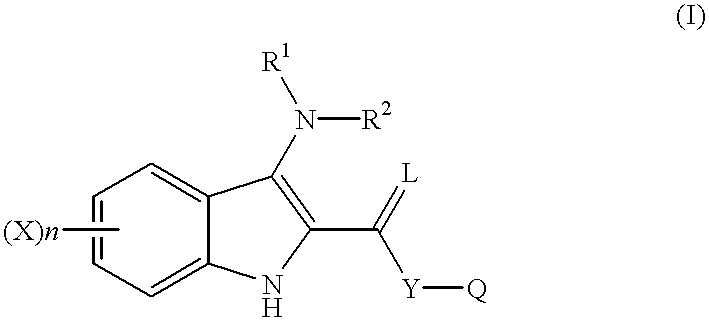
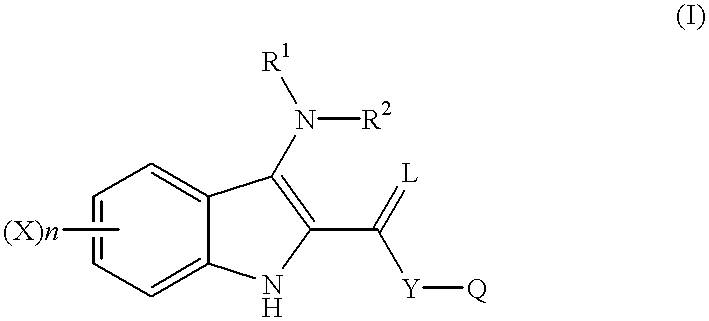
Indole compounds as COX-2 inhibitors
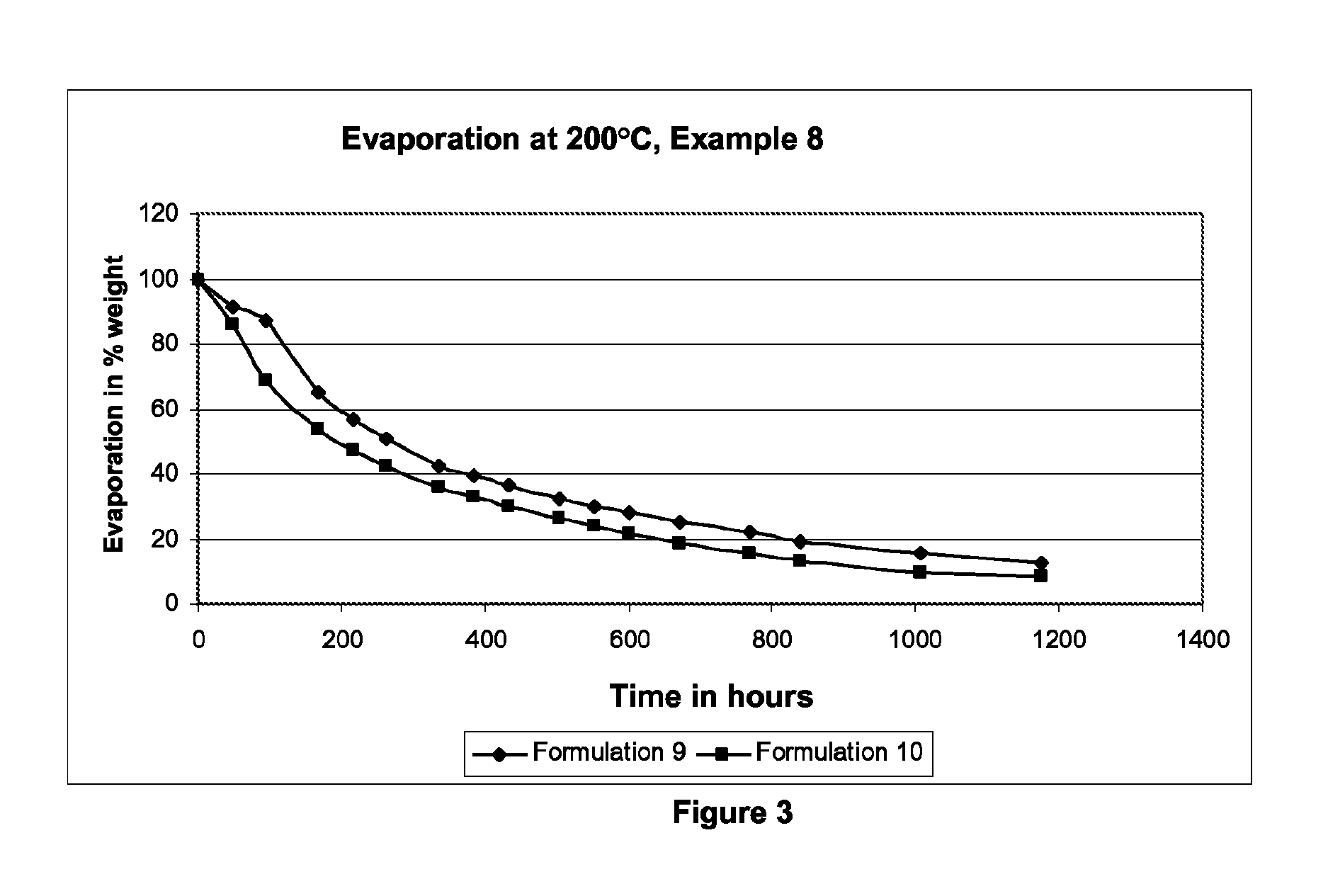
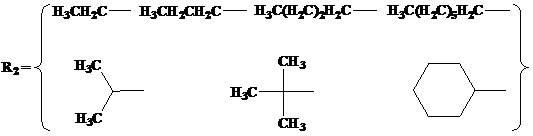
InactiveUS6300363B1Inhibit prostaniod-induced smooth muscle contractionInhibit synthesisBiocideOrganic chemistryDiseaseEthyl group
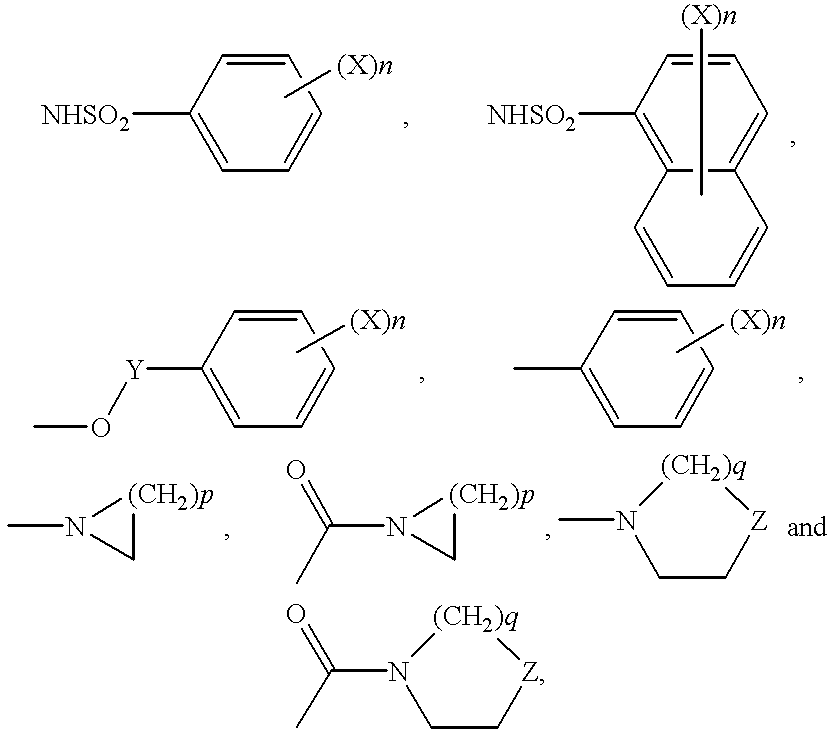
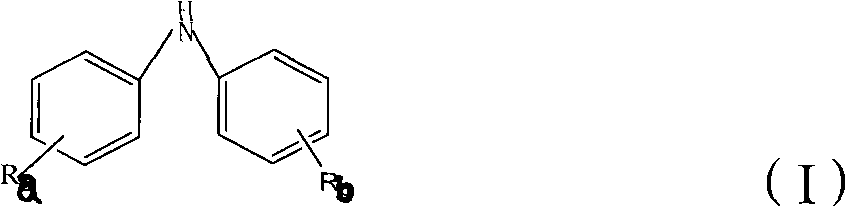
This invention provides a compound of the following formula:and the pharmaceutically acceptable salts thereof, wherein L is oxygen or sulfur; Y is a direct bond or C1-4 alkylidene; Q is C1-6 alkyl, C3-7 cycloalkyl, phenyl, naphthyl, heteroaryl or the like; R1 is hydrogen, C1-6 alkyl or the like; R2 is hydrogen, C1-4 alkyl, C(O)R5 wherein R5 is C1-22 alkyl or C2-22 alkenyl, halosubstituted C1-8 alkyl, halosubstituted C2-8alkenyl, -Y-C3-7 cycloalkyl, -Y-C3-7 cycloalkenyl, phenyl, naphthyl, heteroaryl or the like; X is halo, C1-4 alkyl, hydroxy, C1-4 alkoxy or the like; and n is 0, 1, 2 or 3, with the proviso that a group of formula -Y-Q is not methyl or ethyl when X is hydrogen; L is oxygen; R1 is hydrogen; and R2 is acetyl.This invention also provides a pharmaceutical composition useful for the treatment of a medical condition in which prostaglandins are implicated as pathogens.
Owner:PFIZER INC +1
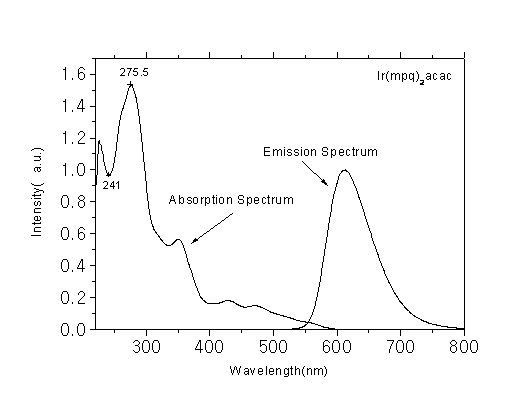
Phosphorescent luminescent materials and preparation method and application thereof
InactiveCN102703059AImprove luminous efficiencyThe synthesis method is simpleGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumQuinoline
The invention discloses phosphorescent luminescent materials and a preparation method and application thereof. The invention is characterized in that the phosphorescent luminescent materials are red light materials containing a metal iridium complex; a structural general formula of the phosphorescent luminescent materials is shown in the specification; and in the structural general formula, R1 and R2 are independently one of alkyl, phenyl, halogen-substituted phenyl, alkyl-substituted phenyl, naphthyl, anthryl, a halogen substituent, methoxy, phenoxy, cyano-substituted carbazolyl, substituted N-phenylcarbazolyl, quinolyl, thiazolyl, thienyl, an aromatic amino group, a group with an azole structure, an aromatic heterocyclic radical, a substituted aromatic heterocyclic radical and a silicon alkyl substituent respectively. The phosphorescent materials have high luminous efficiency; and the high luminous efficiency shows that the compounds can be taken as luminescent materials or main luminescent materials and applied to electroluminescent devices. Through data test and comparison, the materials are organic electroluminescent materials which have excellent performance and a good prospect, and particularly are phosphorescent red light materials which have good performance; and moreover, a method for synthesizing the luminescent materials is simple and low in cost.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Compound for organic electroluminescent device and organic electroluminescent device having the same
ActiveUS20110309345A1Group 4/14 element organic compoundsGroup 5/15 element organic compoundsArylCarbazole
The present invention provides a compound of formula (I) for an organic electroluminescent device:wherein X and Y are each independently selected for the group consisting of an alkyl substituted, aryl substituted or unsubstituted carbazole, indolocarbazole, triphenylsilyl and diphenylphosphine oxide represented by formula (A), (B), (C), (D) or (E),in which R1, R2, and R3 are each independently selected from the group consisting of a hydrogen, an alkyl having 1 to 15 carbons atoms, an aryl group having 6 to 15 carbons atoms, an alkyl substituted, an aryl substituted or unsubstituted triphenylsilyl, and a diphenylphosphine oxide represented by the formula (D) or (E); m and n are each independently 0 or 1, provided that m+n is 1 or more; and Ar1 and Ar2 are each independently selected from the group consisting of an alkyl substituted, aryl substituted or unsubstituted phenyl, tolyl, naphthyl, fluorenyl, anthracenyl, and phenanthryl.
Owner:E RAY OPTOELECTRONICS TECH
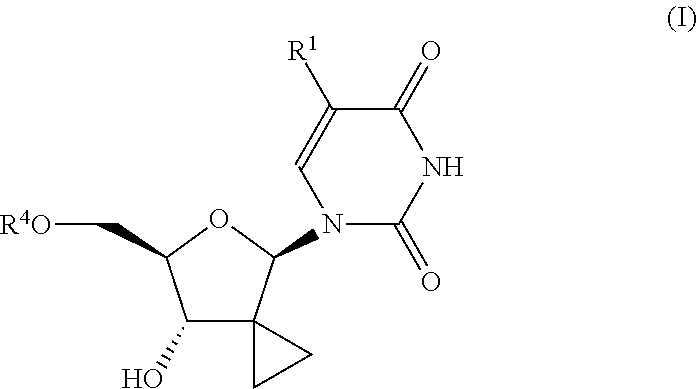
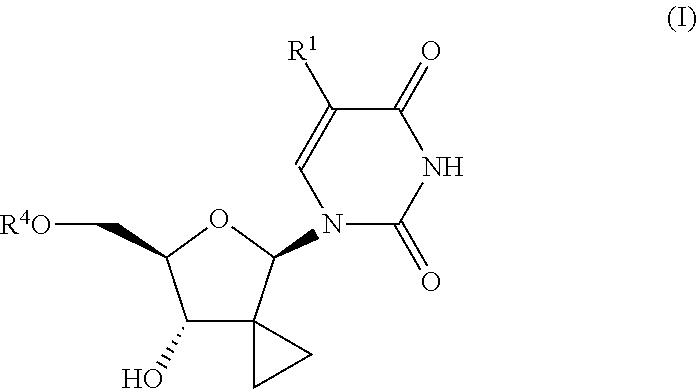
Uracyl cyclopropyl nucleotides
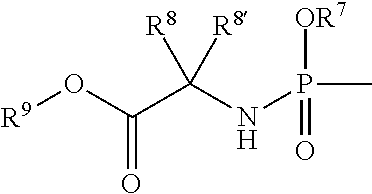
Compounds of the formula I:including any possible stereoisomers thereof, wherein:R1 is hydrogen or halo;R4 is a monophosphate, diphosphate or triphosphate ester; or R4 is a group of formulaR7 is optionally substituted phenyl; naphthyl; indolyl or N—C1-C6alkyloxycarbonyl-indolyl;R8 is hydrogen, C1-C6alkyl, benzyl;R8′ is hydrogen, C1-C6alkyl, benzyl; orR8 and R8′ together with the carbon atom to which they are attached form C3-C7cycloalkyl;R9 is C1-C10alkyl, benzyl, or optionally substituted phenyl;or a pharmaceutically acceptable salt or solvate thereof.pharmaceutical formulations and the use of compounds I as HCV inhibitors.
Owner:JANSSEN PROD +1
Diarylamine novolac resin
ActiveUS20140235059A1Good resist patternInhibition reflexPhotomechanical apparatusSemiconductor/solid-state device manufacturingDevice materialPhenyl group
A novel diarylamine novolac resin such as a phenylnaphthylamine novolac resin, and further a resist underlayer film-forming composition in which the resin is used in a lithography process for manufacturing a semiconductor device. A polymer including a unit structure (A) of Formula (1):(in Formula (1), each of Ar1 and Ar2 is a benzene ring or a naphthalene ring). A method for manufacturing a semiconductor device, including: forming an underlayer film on a semiconductor substrate with the resist underlayer film-forming composition; forming a hardmask on the underlayer film; forming a resist film on the hardmask; forming a resist pattern by irradiation with light or an electron beam followed by development; etching the hardmask with the resist pattern; etching the underlayer film with the hardmask thus patterned; and processing the semiconductor substrate with the underlayer film thus patterned.
Owner:NISSAN CHEM IND LTD
Polymerizable compound, polymerizable composition and liquid crystal display device
ActiveUS20150102259A1High polymerization reactivityWide temperature rangeLiquid crystal compositionsOrganic chemistryAnthraceneLiquid-crystal display
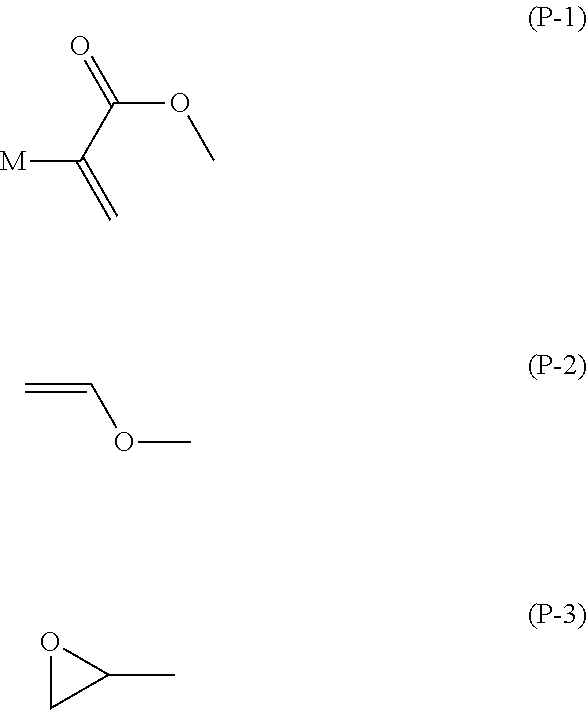
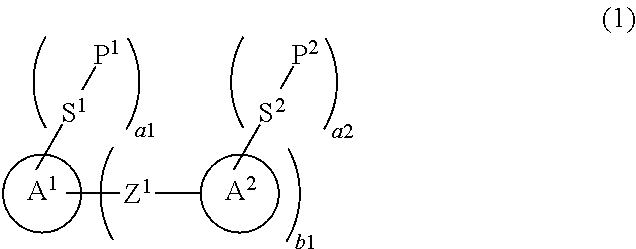
An object is to provide a liquid crystal compound having high polymerization reactivity, a high conversion ratio and high solubility in a liquid crystal composition, a polymerizable composition containing the compound, a liquid crystal composite prepared using the composition, and a liquid crystal display device having the composite. A solution is a polymerizable compound represented by formula (1).In formula (1), P1 and P2 are identically group (P-1), (P-2) or (P-3), and in formula (P-1), M is hydrogen, fluorine, —CH3 or —CF3;S1 and S2 are a single bond, alkylene having 1 to 6 carbons or the like; a1, a2 and b1 are 0, 1, 2, 3 or 4, and a sum of al and a2 is 4; ring A1 is naphthalene, anthracene or phenanthrene; and ring A2 is cyclohexyl, phenyl, naphthyl, anthracenyl or phenanthrenyl; and Z1 is a single bond, —CO—, —COO— or the like.
Owner:JNC CORP +1
Meteorologic balloon and production method thereof
ActiveCN101592742AThere will be no problem of uneven distributionAgainst destructive powerInstrumentsPotassium hydroxideDioctyl sebacate
The invention discloses a meteorologic balloon and a production method thereof. The raw materials consist of a solid raw material, an oily raw material and a gluey raw material; the solid raw material comprises the following components by weight: 0 to 0.30 portion of potassium hydroxide, 0 to 0.15 portion of casein, 0 to 0.10 portion of peregal O, 1.00 to 3 portions of sulfur, 1.00 to 2 portions of N-phenyl-2-naphthylamine, 1.00 to 2 portions of N-phenyl-N'-isopropyl-ursol, 1.50 to 3 portions of ethyl-phenyl-zinc dithiocarbamate, 0.20 to 5 portions of zinc oxide, and 5 to 30 portions of chloroprene polymer; the oily raw material is dioctyl sebacate, and the weight rate of the dioctyl sebacate is 5.00 to 10.0 portions; and the gluey raw material is centrifugally-concentrated natural latex, and the adding amount of the centrifugal concentrated natural latex is 100 portions. The weight of the meteorologic balloon is 1,600 to 1,700 grams, the length of the balloon body is 3.05 to 3.55 meters, the width of the ball handle is 10 to 11 centimeters, and the length of the ball handle is 12 to 16 centimeters. The meteorologic balloon can adapt to the requirement of probing high-altitude meteorological elements of more than 35,000 meters, has the advantages of good aging resistance, high blastoff height, long balloon service time and the like, and can effectively overcome the morning-evening difference.
Owner:CHEMCHINA ZHUZHOU RUBBER RES & DESIGN INST
Use of an oligomer-based additive for stabilizing a lubricating composition for a conveyor chain
The use of an additive including a mixture of oligomers that is produced from the reaction of aromatic amines chosen from:(i) the reaction with one another of diphenylamine (DPA) compounds of formula (I) below:wherein the groups R1 and R2 stand for, independently of one another, a hydrogen or a linear or branched alkyl group having from 1 to 30 carbon atoms, advantageously from 4 to 12 carbon atoms,(ii) the reaction with one another of phenyl-α-naphthylamine (PAN) compounds of formula (II) below:wherein the group R3 stands for a hydrogen or a linear or branched alkyl group having from 1 to 30 carbon atoms, advantageously from 4 to 12 carbon atoms, and(iii) the reaction of a (DPA) compound of formula (I) above with a (PAN) compound of formula (II) above as an agent for the stabilization of a lubricating composition for a conveyor chain subjected to a temperature of at least 120° C.
Owner:NYCO CO LTD
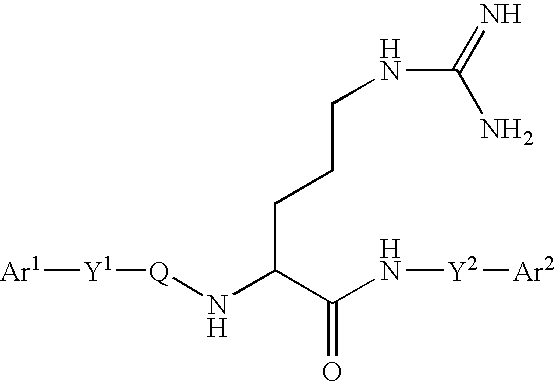
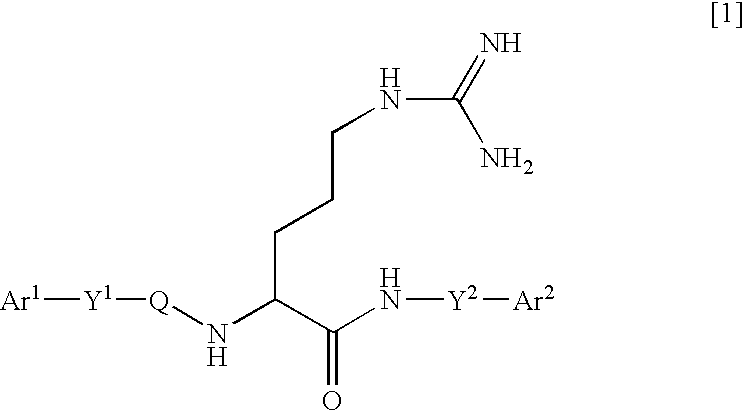
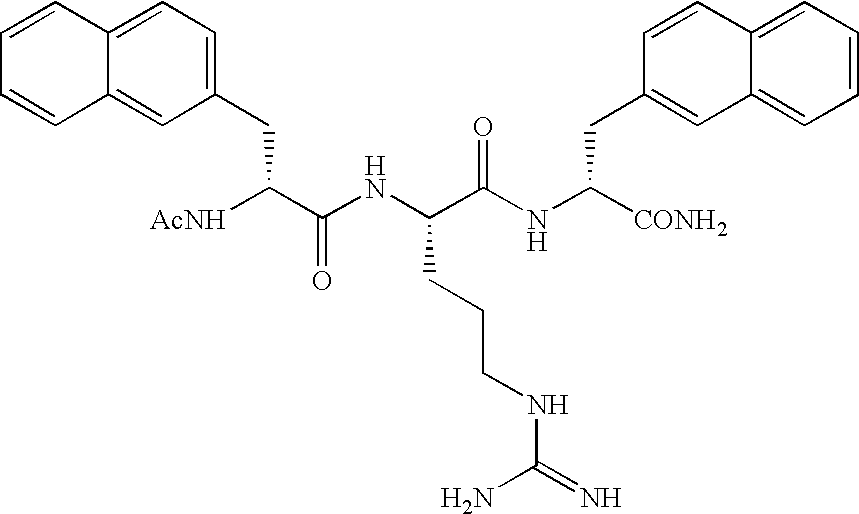
Arginine derivatives
An arginine derivative represented by the formula: [wherein Ar<1 >and Ar<2 >may be the same or different, and are each a phenyl group, a substituted phenyl group, a naphthyl group, a substituted naphthyl group or a heteroaromatic ring group containing one or more of nitrogen, oxygen and sulfur atoms; Y<1 >is a C1-5 alkylene group, a C2-5 alkenylene group or a single bond; and the C1-5 alkylene group optionally contains a carbon atom substituted with a phenyl group, a substituted phenyl group, a naphthyl group, a substituted naphthyl group or a C1-10 acylamino group; Q is a carbonyl group or a sulfonyl group; Y<2 >is a C1-5 alkylene group; the C1-5 alkylene group optionally contains a carbon atom substituted with a phenyl group, a substituted phenyl group, a naphthyl group, a substituted naphthyl group, a hydroxyl group, a carbamoyl group, a mono-C1-5 alkylamide group or a di-C1-5 alkylamide group], or a pharmaceutically acceptable salt thereof. There are provided peptidergic ligands which have the affinity and specificity to MC4 receptor.
Owner:TAISHO PHARMACEUTICAL CO LTD
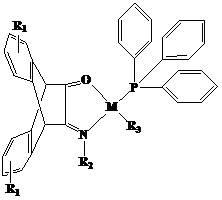
N,O-single ligand metal catalyst with stereochemical structure and preparation method thereof
The invention relates to an N,O-single ligand metal catalyst with a stereochemical structure shown in the description and a preparation method thereof. The catalyst has the following structure general formula, wherein M represents a nickel, palladium, cobalt, iron or copper central metal atom; R1 represents bromine, chlorine, iodine, methyl, propyl, butyl, phenyl, naphthyl, methoxy or ethyoxyl; R2 represents aromatic amine or fatty amine; and R3 represents phenyl, naphthyl, bromine or methyl. The catalyst disclosed by the invention has high light transmission, high glass-transition temperature, excellent thermal stability and oxidation stability, low moisture rate, low permittivity and excellent processability, solubility property and blocking property.
Owner:横峰县虹联铝业有限公司
1-Substituted 4-carbamoyl-1,2,4-triazol-5-one derivatives and herbicide
InactiveUS6077814AImprove herbicidal activityEasy to synthesizeBiocideOrganic chemistryPhytotoxicityAcyl group
PCT No. PCT / JP98 / 00803 Sec. 371 Date Aug. 23, 1999 Sec. 102(e) Date Aug. 23, 1999 PCT Filed Feb. 26, 1998 PCT Pub. No. WO98 / 38176 PCT Pub. Date Sep. 3, 1998A 1-phenyl, naphthyl or aralkyl-4-(N,N-di-substituted carbamoyl)-1,2,4-triazol-5-one derivative represented by the general formula (I) wherein A is an unsubstituted or substituted phenyl group, 1-naphthyl group, 5,6,7,8-tetrahydro-1-naphthyl group or an aralkyl group such as an unsubstituted or substituted benzyl group and so on, R1 is a lower alkyl group and so on, and R2 is an unsubstituted or substituted phenyl group and so on, was prepared as novel compounds. This 1-substituted-4-carbamoyl-1,2,4-triazol-5-one derivative does not substantially exhibit phytotoxicity to various agricultural crop plants and therefore is useful as a selective herbicide.
Owner:HOKKO CHEM IND CO LTD (JP)
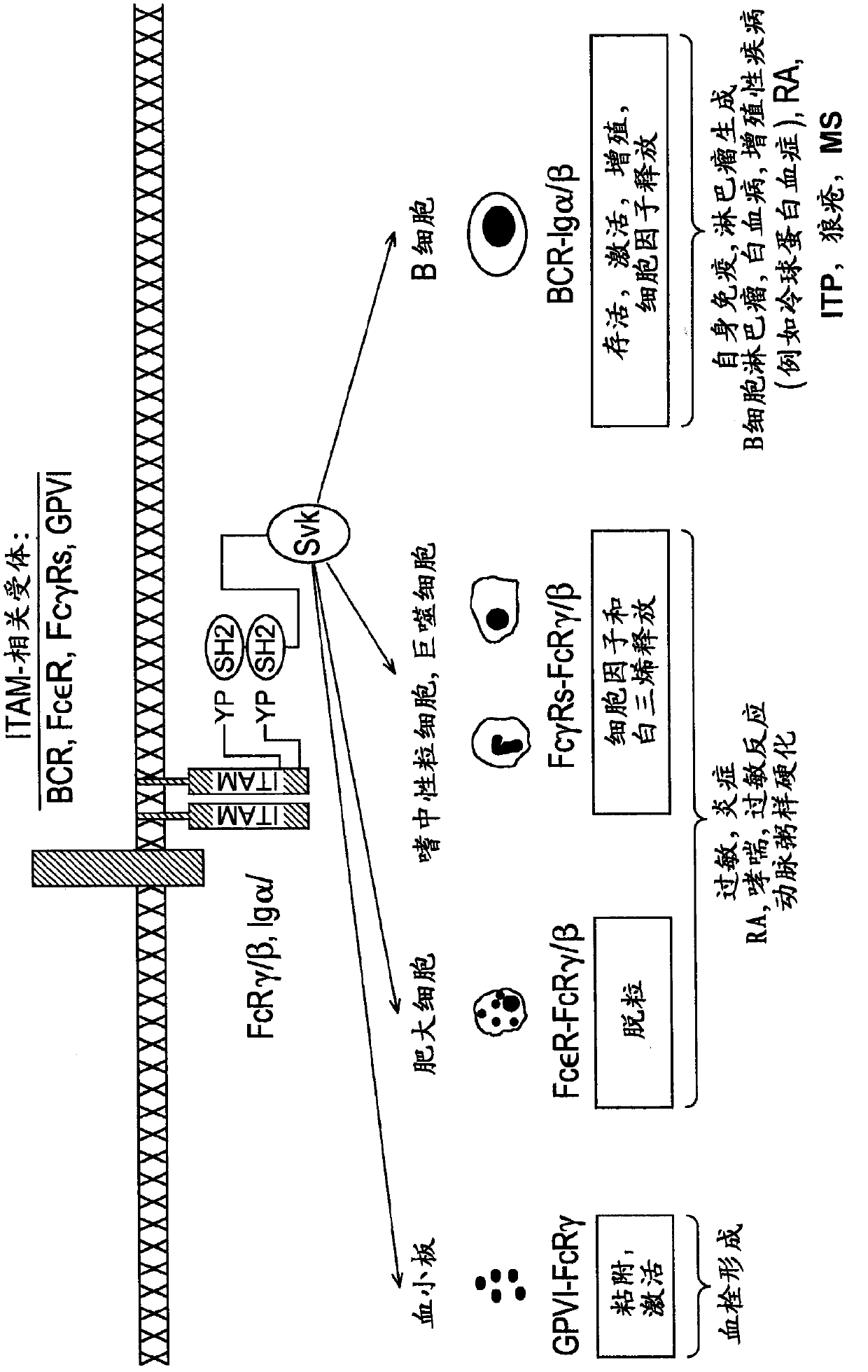
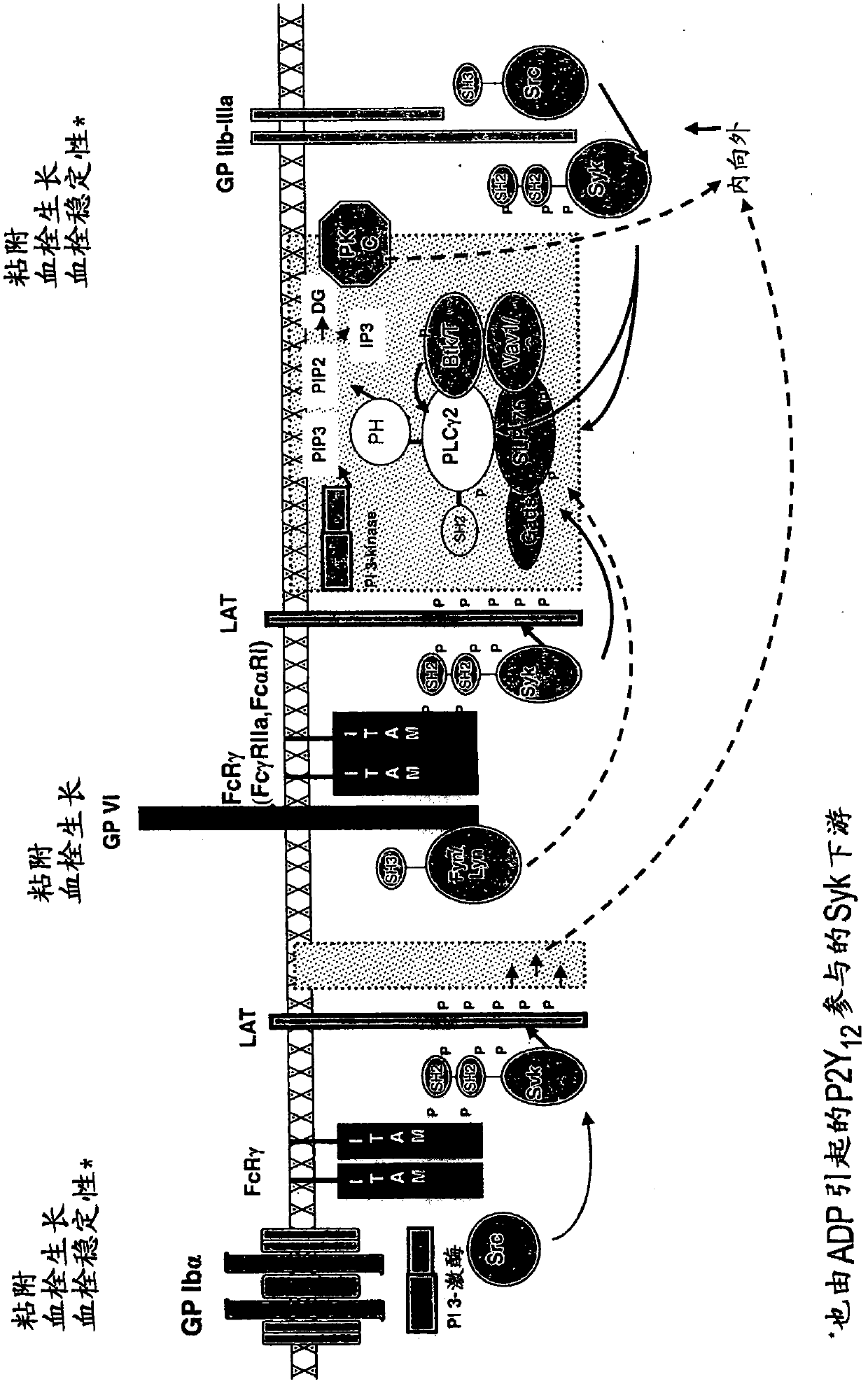
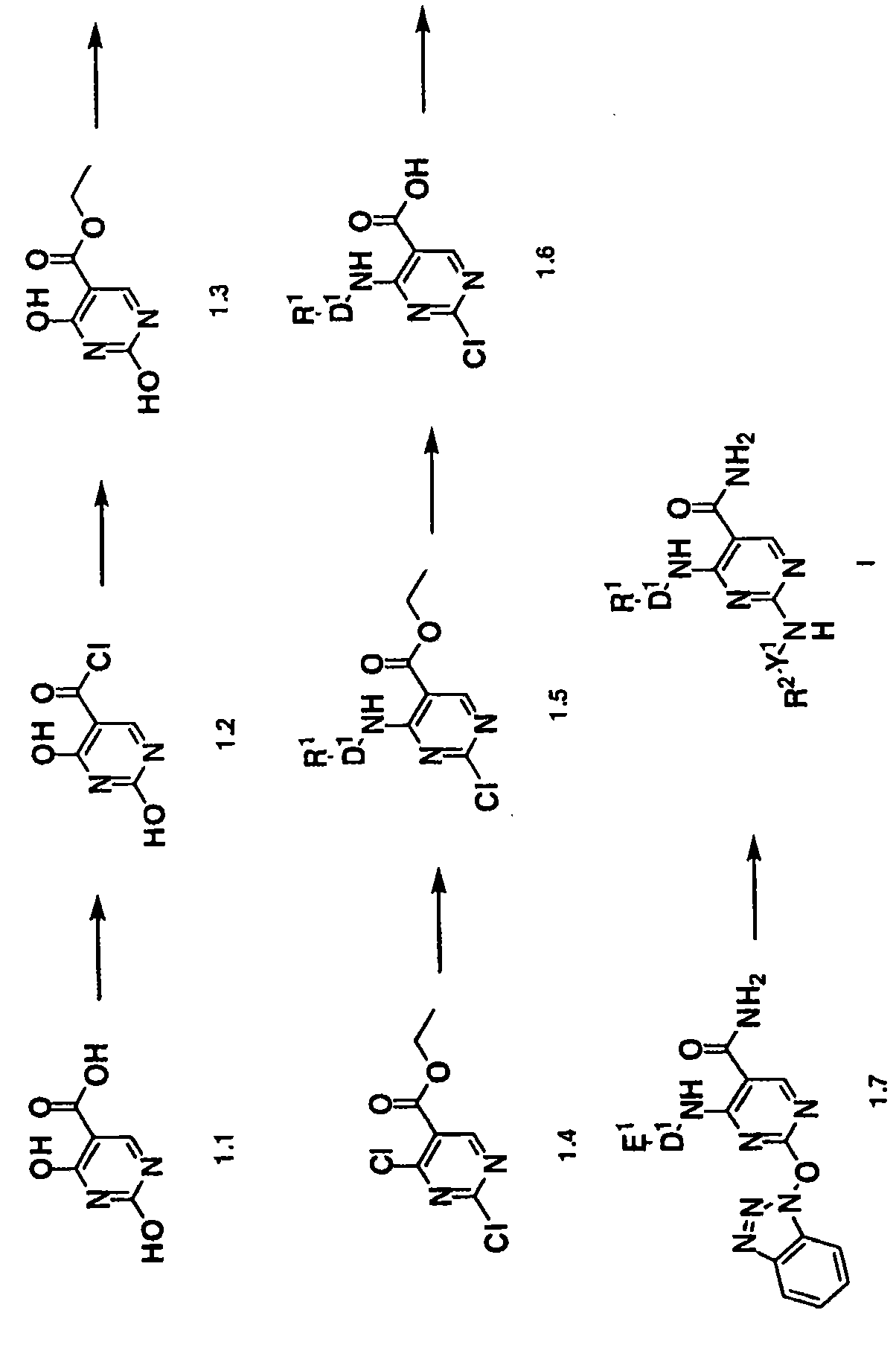
2, 6-diamino- pyrimidin- 5-yl-carboxamides as SYK or JAK kinases inhibitors
The present invention is directed to compounds of formula I-V and tautomers thereof or pharmaceutically acceptable salts, esters, and prodrugs thereof which are inhibitors of syk kinase. The present invention is also directed to intermediates used in making such compounds, the preparation of such a compound, pharmaceutical compositions containing such a compound, methods of inhibition syk kinase activity, methods ofinhibilion the platelet aggregation, and methods to prevent or treat a number of conditions mediated at least in part by syk kinase activity, such as cardiovascular disease, inflammatory disease and autoimmune disease and cell proliferative disorder. Formula (I) Y1 is selected from the group consisting of: (a) and (b); Z is O or S; D1 is selected from the group consisting of: (a) phenyl substituted with a group, R5 (b) naphthyl (c) C3-8cycloalkyl (d) heteroaryl (e) heterocyclyl.
Owner:PORTOLA PHARMA INC
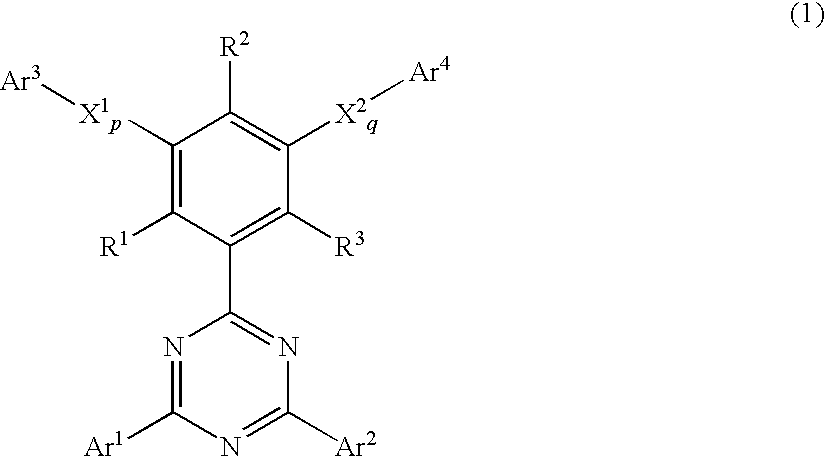
Phenyl-substituted 1,3,5-triazine compound, process for producing the same, and organic electroluminescent device containing the same as component
ActiveUS8268997B2Outstanding propertyDischarge tube luminescnet screensOrganic chemistry methods1,3,5-TriazineHydrogen atom
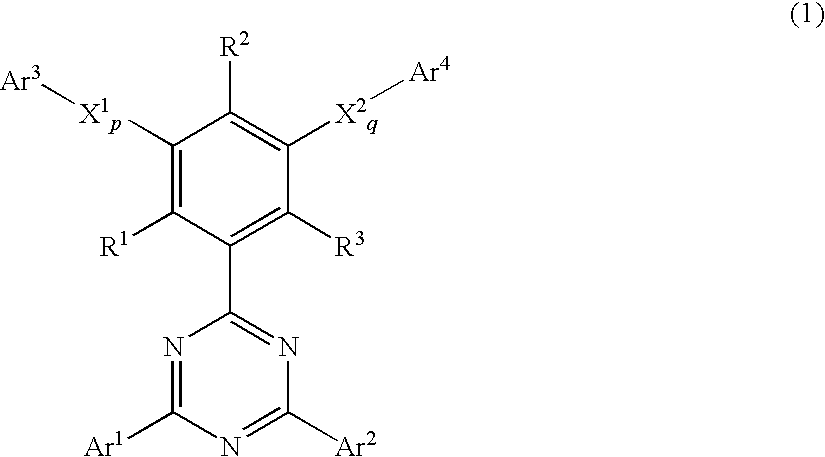
A phenyl-substituted 1,3,5-triazine compound represented by the general formula (1);wherein Ar1 and Ar2 independently represent substituted or unsubstituted phenyl, naphthyl or biphenylyl group; R1, R2 and R3 independently represent hydrogen atom or methyl group; X1 and X2 independently represent substituted or unsubstituted phenylene, naphthylene or pyridylene group; p and q independently represent an integer of 0 to 2; and Ar3 and Ar4 independently represent substituted or unsubstituted pyridyl or phenyl group. This compound is suitable for an organic electroluminescent device.
Owner:SAGAMI CHEM RES CENT +1
4-aroyl-1,8-naphthalimide compound and preparation method and use thereof
InactiveCN104003935AReduce manufacturing costNovel synthetic routeOrganic chemistryMaterial analysis by observing effect on chemical indicatorAcyl groupOrganosolv
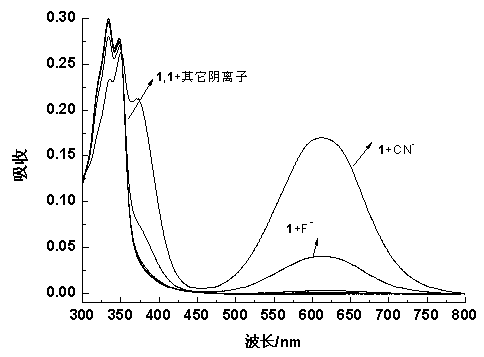
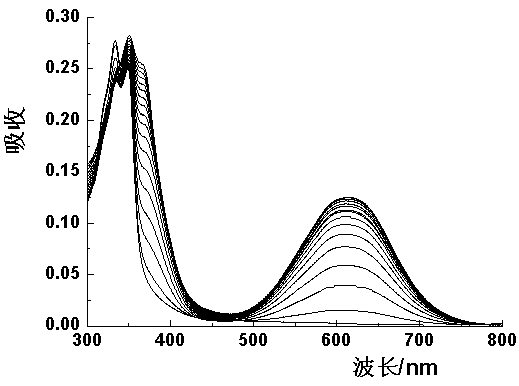
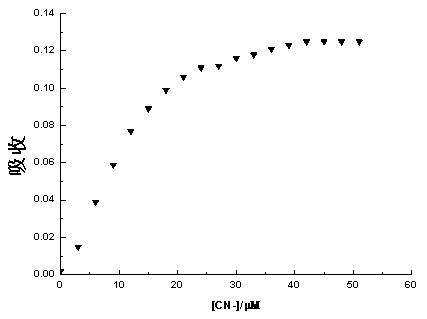
The invention discloses a 4-aroyl-1,8-naphthalimide compound and a preparation method and use thereof, wherein the 4-aroyl-1,8-naphthalimide compound has a structural formula as shown in the specification, R1 is C1-C10 straight chain or branched chain alkyl; and R2 is phenyl, naphthyl, biphenylyl, substituted phenyl, quinary or senary heteroaryl or benzo quinary or senary heteroaryl. The preparation method is as follows: a 4 bromo-1,8-naphthalimide compound is used as a raw material to react with substituted phenylacetonitrile or aromatic ring acetonitrile in an organic solvent in the presence of an alkali catalyst to obtain a 4-aryl acetonitrile-1,8-naphthalimide compound, and then the 4-aroyl-1,8-naphthalimide compound is obtained in the effects of fluoride ions or cyanide ions. The 4-aryl acetonitrile-1, 8-naphthalimide compound is used as a color or fluorescence sensor for detection of the fluoride ions or cyanide ions, and has high sensitivity and high selectivity during identifying of the cyanide ions in a mixed solvent.
Owner:SHANGHAI INST OF TECH
LDL receptor gene expression promoters
InactiveUS6159974AReduced activityEasy to synthesizeBiocideOrganic chemistryReceptorTriazine derivative
PCT No. PCT / JP98 / 01225 Sec. 371 Date Sep. 24, 1999 Sec. 102(e) Date Sep. 24, 1999 PCT Filed Mar. 23, 1998 PCT Pub. No. WO98 / 42686 PCT Pub. Date Oct. 1, 1998LDL receptor gene expression promoters containing a 1,2,4-triazine derivative of the formula (I): wherein R1 is optionally substituted phenyl or heterocycle; R2 is optionally substituted phenyl, naphthyl, aralkyl, 5- or 6-membered aromatic heterocycle, alkyl, or alkenyl; X is O, S or NR4; R3 is optionally substituted phenyl, naphthyl, aralkyl, 5- to 6-membered aromatic heterocycle, alkyl, cycloalkyl or alkenyl, provided that when X is NR4, then it may form an optionally substituted nitrogen-containing heterocycle, or a pharmaceutically acceptable salt thereof, which can increase the expression dose (the amount of mRNA) of LDL receptor gene, and thus increase the amount of LDL receptor, and reduce the serum cholesterol level, and are useful in the treatment of hyperlipidemia.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
2,6-diphenyl naphthalene derivative and preparation method and application thereof
ActiveCN106083606AImprove external quantum efficiencyImprove luminous efficiencyAmino preparation from aminesOrganic compound preparationOrganic electroluminescenceOptoelectronic materials
The invention provides a 2,6-diphenyl naphthalene derivative and a preparation method and application thereof, and relates to the technical field of organic optoelectronic materials. The 2,6-diphenyl naphthalene derivative obtained by optimizing the molecular structure design has the higher optical extraction efficiency, can be used for preparing organic electroluminescence devices and especially can effectively improve the optical emitting efficiency of OLED devices by serving as an optical extraction material of the organic electroluminescence devices, and the OLED devices are superior to existing commonly-used OLED devices. The invention further provides the preparation method of the 2,6-diphenyl naphthalene derivative. The preparation method is simple, and the raw materials are easy to obtain.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Benzoheterocyclic compound, display panel and display device
ActiveCN109293645AImprove light extraction efficiencyAvoid loss of color purityOrganic chemistrySolid-state devicesChemistryPhenyl group
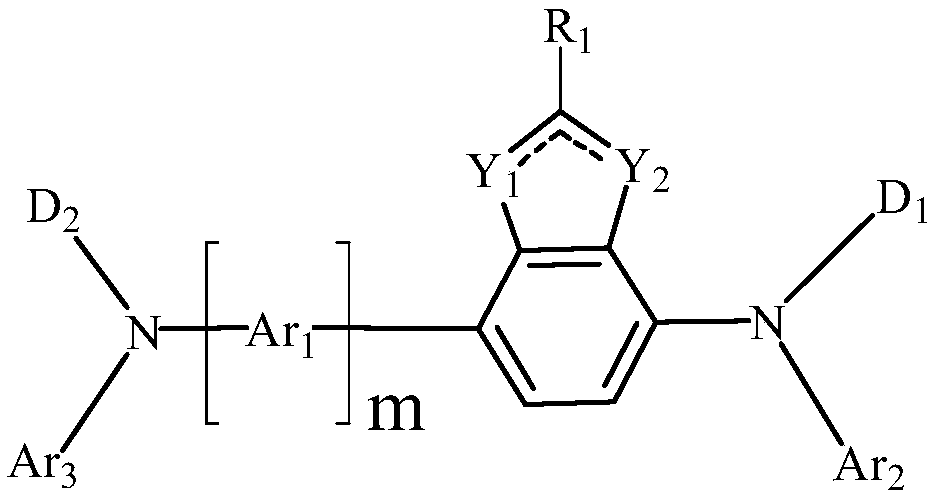
The invention provides a benzoheterocyclic compound. The benzoheterocyclic compound has a structure represented by formula (I); and in the formula, R1 is any one of hydrogen atom, a C1-C20 alkyl group, a C1-C20 alkoxy group, a phenyl group, a naphthyl group, an anthryl group and a phenanthryl group, Y1 and Y2 are respectively independently selected from a carbon atom, an oxygen atom and a sulfur atom, one of the Y1 and Y2 is the carbon atom, Ar1 is a single-bond or monosubstituted or unsubstituted group of any one of a phenyl group, a naphthyl group, a fluorenyl group, a phenanthryl group, anacenaphthenyl group and an aromatic heterocyclic ring, m is 0 or 1, D1 and D2 are respectively independently selected from an aryl group or a heteroaryl group, and Ar2 and Ar3 are respectively independently selected from an aryl group and a heteroaryl group. The benzoheterocyclic compound can be used as a material for a capping layer (CPL) of an OLED device to obtain a high light extraction efficiency and avoid the problem of reduction of the color purity of the blue light-emitting device.
Owner:WUHAN TIANMA MICRO ELECTRONICS CO LTD
Composite amine antioxidant, preparation and use thereof
The invention provides a composite amine antioxidant, which at least comprises the following two substances: (1) alkylated diphenylamine and (2) alkylated N-phenyl-alpha-naphthylamine. The composite amine antioxidant is prepared by addition of diisobutylene into mixture of the diphenylamine and the N-phenyl-alpha-naphthylamine for alkylation reaction in the presence of acid catalyst. The composite amine antioxidant provided by the invention has good oil-soluble performance and antioxidation, and can be widely applied to various lubricating oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
4-(substituted-1,3-diyne)-4-(trifluoromethyl)-3,4-dihydro substituted quinazoline-2-ketone compound as well as preparation method and application thereof
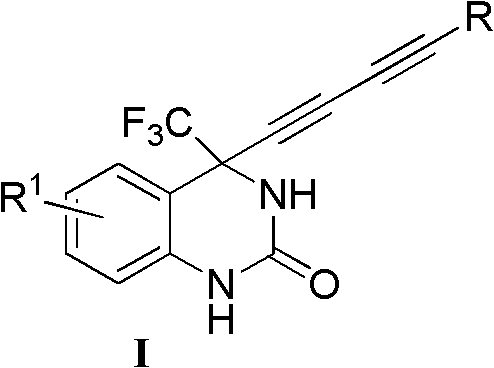
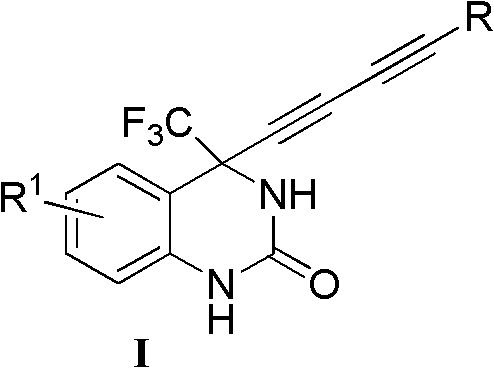
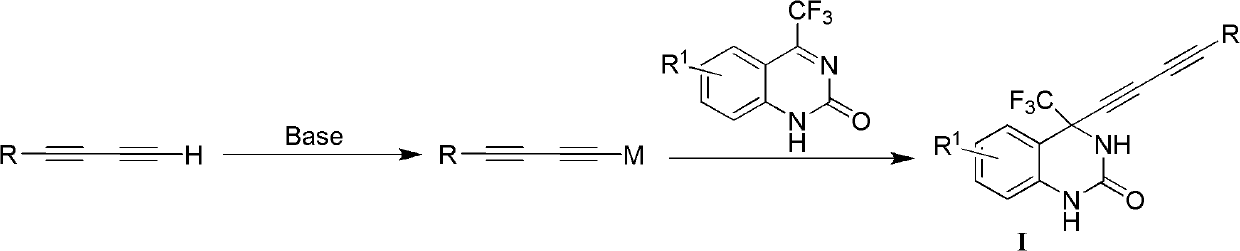
InactiveCN102060786AThe synthesis method is simple and feasibleHigh yieldOrganic active ingredientsOrganic chemistryFuranOrganosolv
The invention relates to a 4-(substituted-1,3-diyne)-4-(trifluoromethyl)-3,4-dihydro substituted quinazoline-2-ketone compound as well as a preparation method and application thereof. The preparation method comprises the following steps of: completely reacting 4-substituted-1,3-diyne with 4-trifluoromethylquinazoline-2-ketone at -78-40 DEG C in an organic solvent under the action of alkali by using a chiral alkamine reagent as a ligand; and washing, extracting and separating to obtain the target product 4-(substituted-1,3-diyne)-4-(trifluoromethyl)-3,4-dihydro substituted quinazoline-2-ketone, wherein the molar ratio of the 4-substituted-1,3-diyne to the alkali to the 4-trifluoromethylquinazoline-2-ketone is (1-3):(1-3):1. The structural general formula of the compound is shown as I, wherein R is a 1-6 carbon alkyl group, a 3-7-membered ring alkyl group or an aromatic group such as a phenyl group, a naphthyl group, a furan group and a pyridyl group; and R1 is halogen on the 5th, 6th, 7th or 8th site, a 1-3 carbon alkyl group or a 1-3 alkoxyl group. The compound can be used for treating virus infections, particularly the infections of HBV (Hepatitis B Virus), HCV (Hepatitis C Virus) or HIV (Human Immunodeficiency Virus).
Owner:TIANJIN UNIV
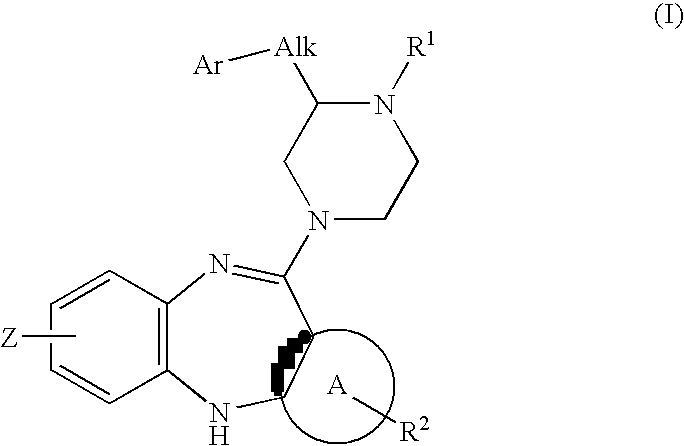
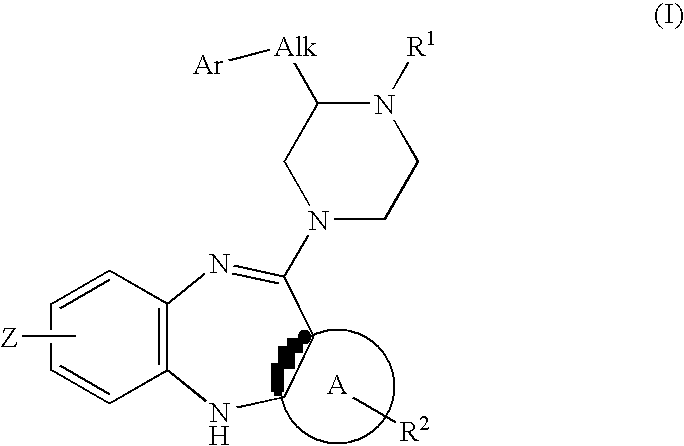
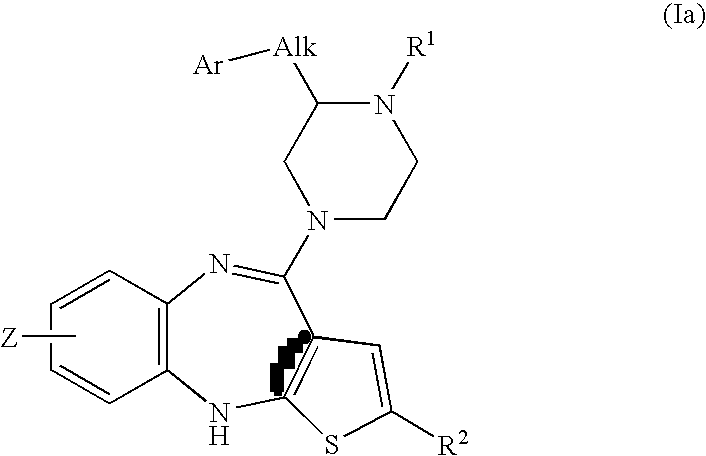
Piperazine substituted aryl benzodiazepines and their use as dopamine receptor antagonists for the treatment of psychotic disorders
Owner:ELI LILLY & CO
Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices
A naphthopyran compound represented by the formulaB1 and B2 are selected from a phenyl, naphthyl, or heterocyclic aromatic group, or may combine to form one or more aromatic rings. B1 and B2 are optionally substituted with one or more substituents. R3, R4, R5, R6, and R10 are selected independently from hydrogen, halogen, —Ra, —OH, —ORa, —O—CO—Ra, —CN, —NO2, —SO2Ra, —SORa, —SH, —SRa, —NH2, —NHRa, —NRaRa, and —NRbRc. Any two or more of R5, R6 or R10 may combine to form a cyclic group. R7 is a mesogenic group containing at least two rings connected to each other through a covalent bond or linking unit. The linking unit is an ester, —Rd—, —O—, —ORd—, —ORdO—, —OCORd—, —OCORdO—, —S—, —CH═CH—, —CH═N—, —C≡C—, or —N═N—, where Rd is a linear or branched (C1-18)alkyl or a linear or branched (C1-18)haloalkyl group. The naphthopyran compound may be incorporated into an optical article.
Owner:ALPHAMICRON INC
Five-membered ring phosphate compound, and preparation method and application thereof
PendingCN111217856AImprove film formationRaise high temperatureGroup 5/15 element organic compoundsSecondary cells servicing/maintenanceElectrolytic agentElectrical battery
The invention belongs to the technical field of flame retardance, and especially relates to a five-membered ring phosphate compound. The structural general formula of the five-membered ring phosphatecompound is represented by formula 1 shown in the description; and in the formula, R<1>, R<2>, R<3>, R<4>, R<5>, R<6>, R<7>, R<8>, R<9>, R<10>, R<11>, R<12>, R<13>, R<14>, R<15>, R<16>, R<17> and R<18> are independently selected from hydrogen, an alkyl group, an alkenyl group, an alkynyl group, an alkoxy group, an alkynyloxy group, an enyloxy group, a silyl group, a siloxane group, aryl silicon, an arylsilyl group, an arylsiloxy group, a halogenated phenyl group, a halogenated biphenyl group, a phenolic group, an alkyl-containing phenolic group, an alkenyl-containing phenolic group, an alkynyl-containing phenolic group, a nitrile-containing phenolic group, a monohalogenated phenolic group and a polyhalogenated phenolic group. The five-membered ring phosphate compound provided by the invention has excellent flame retardancy, can effectively prevent electrolytes from being oxidized when being applied to the field of batteries, reduces oxygenolysis of the electrolytes on positive electrodes, and remarkably improves the comprehensive properties of high temperature, circulation, storage and the like of the batteries.
Owner:EVERGRANDE NEW ENERGY TECH SHENZHEN CO LTD
Oil composition for copper foil rolling and uses thereof
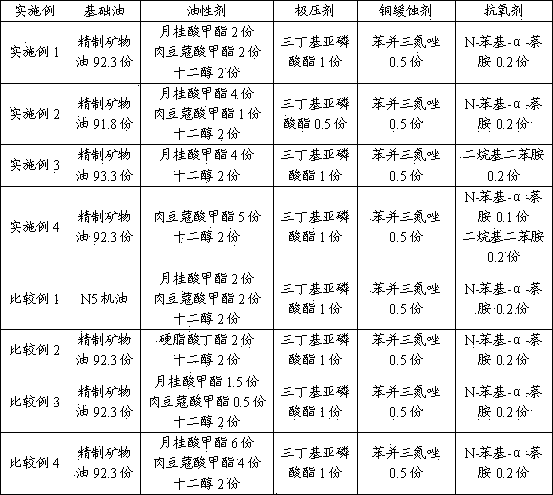
InactiveCN104342247AGood lubricating and cooling effectImprove anti-corrosion and anti-rust performanceWork treatment devicesMetal rolling arrangementsPhosphoric Acid EstersEngineering
The invention relates to an oil composition for copper foil rolling and uses thereof, mainly overcoming the problems of the prior art, namely poor lubrication performance, much oil mist and poor low-temperature annealing detergency. The oil composition adopts a mixture comprising following components by weight: a) 90-98 parts of base oil; b) 1-10 parts of an oiliness agent; c) 0.5-4 parts of an extreme pressure agent; d) 0.05-1 part of a copper corrosion inhibitor; and e) 0.05-1 part of an antioxidant, wherein the base oil is selected from purified narrow-fraction dearomatization solvent oil; the oiliness agent is selected from C12-C14 fatty acid methyl ester and C10-C14 alcohol; the extreme pressure agent is selected from tributylphosphite; the copper corrosion inhibitor is selected from benzotriazole and derivatives thereof; and the antioxidant is at least one of N-phenyl-alpha-naphthylamine or dialkyldiphenylamine. By adoption of the abovementioned technical scheme, the problems are solved well, and the oil composition can be used for process lubrication in a copper foil cold-rolling process.
Owner:CHINA PETROLEUM & CHEM CORP
Polyalkylene glycol-based industrial lubricant compositions
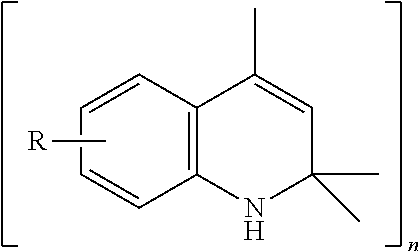
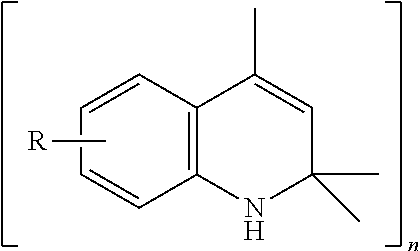
ActiveUS20160083671A1Poor oxidation protectionSuperior oxidation protectionOrganic chemistryAdditivesMetal working fluidQuinoline
A lubricant composition comprises as a lubricant base, an oil soluble polyalkylene glycol suitable for use as a lubricant in an industrial oil, grease or metal working fluid; and an additive comprising (1) alkylated phenyl-α-naphthylamine; and (2) 2,2,4-trialkyl-1,2-dihydroquinoline.
Owner:VANDERBILT CHEM LLC
Compound, display panel and display device
ActiveCN110078755AImprove stabilityExtend your lifeSilicon organic compoundsSolid-state devicesAcenaphthyleneBenzanthracenes
The invention provides a compound, a display panel and a display device. The compound has the structure shown in formula (I), L represents substituted or unsubstituted phenyl, naphthyl, pyridyl, pyrimidinyl and pyrazinyl; D is an electron-donating group and independently selected from any one of substituted or unsubstituted phenyl, biphenyl, naphthyl, anthryl, phenanthryl, acenaphthylene, pyrenyl,peryl, flurenyl, spiro bifluorenyl, benzophenanthryl, benzanthracene, fluoranthryl, picene, furyl, benzofuryl, dibenzofuryl, thienyl, benzothienyl, dibenzothienyl, phenoxazine, thianthryl, carbazolyl, acridinyl and diarylamino. The designed compound has a TADF characteristic and can emit light by triplet exciton of traditional fluorescence molecular transition inhibition so as to improve the device efficiency.
Owner:WUHAN TIANMA MICRO ELECTRONICS CO LTD
Phosphorescent compound and organic light emitting diode device using same
ActiveCN109678867AHigh color purityIncrease brightnessOrganic chemistrySolid-state devicesPhenanthrolineStructural formula
The invention relates to a phosphorescent compound and an organic light emitting diode device using the same, in particular to a soluble phosphorescent host compound with excellent color purity, highluminance and luminous efficiency and an OLED device using the same. The phosphorescent compound is characterized in that the structural formula is shown as formula I (please see the specification forthe formula), and in the structural formula I, Z is independently selected from the following structures (please see the specification for the formulas), wherein Ar is independently selected from C6-C30 aryl groups and C2-C30 heteroaryl groups, the C6-C30 aryl groups are selected from one of a phenyl group, a naphthyl group, a biphenyl group, a terphenyl group and a phenanthryl group, and the C2-C30 heteroaryl groups are selected from one of a pyridyl group, a bipyridyl group, a quinolyl group, an isoquinolyl group, a phenanthroline group and a triazinyl group. The phosphorescent compound adopts the chemical formula I as a luminous layer of the organic light emitting diode device, and has the excellent color purity and luminance as well as an extended durability effect.
Owner:ZHEJIANG HUADISPLAY OPTOELECTRONICS CO LTD
Organic material and application thereof in organic electroluminescence devices
ActiveCN101875637BHigh electron mobilityOrganic chemistrySolid-state devicesElectron transporting layerOrganic electroluminescence
The invention relates to an organic material and an organic electroluminescence device comprising the same. The material has the structural general formula shown in the description, wherein Ar is sub-fused-ring aromatic hydrocarbons with the carbon atoms between 6 and 30, or sub-fused heterocyclic aromatic hydrocarbons with the carbon atoms between 6 and 30; R1 is phenyl, biphenyl, naphthyl, alkyl with 1 to 5 carbon atoms or hydrogen; and n is an integer from 2 to 3. The organic material can be used as an electron transport layer in the organic electroluminescence device.
Owner:KUNSHAN VISIONOX DISPLAY TECH +2
Wear-resisting pressure-proof rubber shock-absorbing block
The invention relates to a wear-resisting pressure-proof rubber shock-absorbing block. A raw material formula of the shock-absorbing block is composed of the following components: tyrene-butadiene rubber (1712), natural rubber, alkyl phenolic resin, ferric chloride, barium sulfate, N-phenyl-beta-naphthylamine, and a cycloparaffin softener. The wear-resisting pressure-proof rubber shock-absorbing block disclosed by the invention is high in compressive strength, good in wear resistance, and applicable to the shock absorption of big-vibration electromechanical devices such as punch presses and fans and the like.
Owner:QINGDAO BOYUTE RUBBER & PLASTIC PRODS
Thermotropic liquid crystal polyester and preparation method thereof
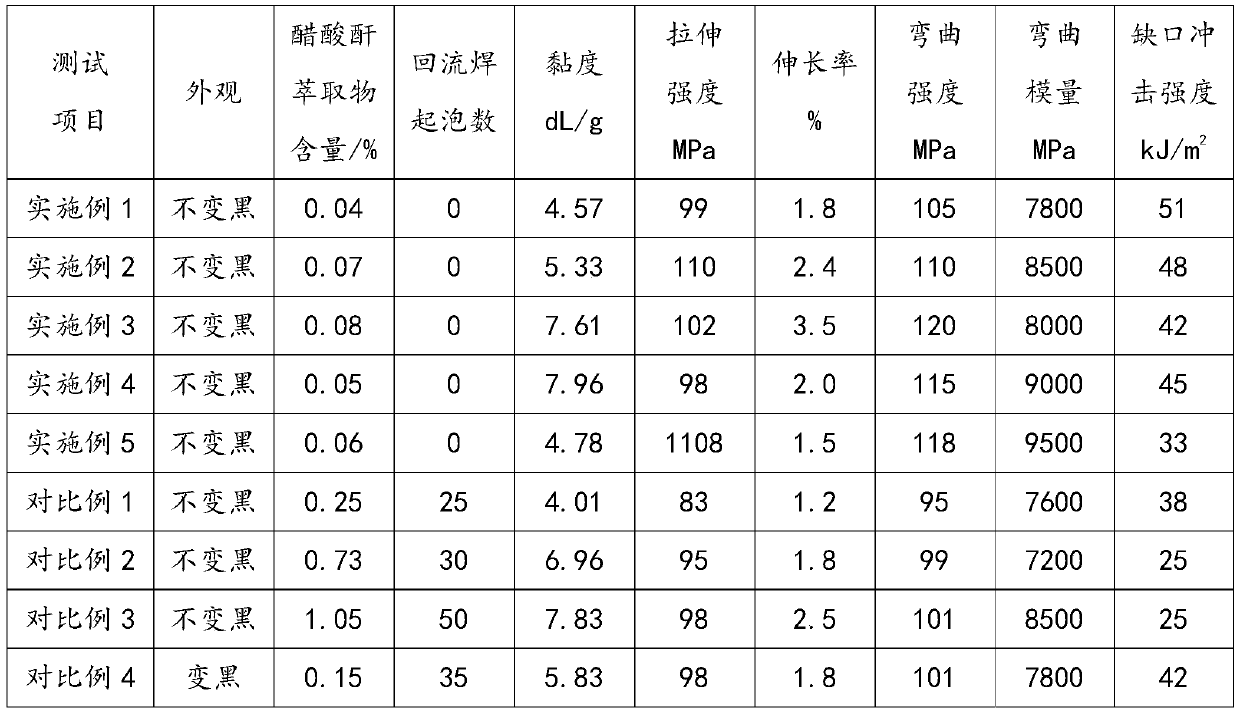
ActiveCN110982050ANo blackeningThe acetylation reaction is completeLiquid crystal compositionsPolyesterPolymer science
The invention belongs to the field of materials, and discloses thermotropic liquid crystal polyester which is obtained by reaction of a monomer, aliphatic anhydride and a catalyst; the monomer comprises at least one of HO-Ar-COOH (I), HO-Ar-OH (II) and HOOC-Ar-COOH (III); Ar is at least one of phenyl, biphenyl, naphthyl, anthryl or phenanthrene; the amount of substance of aliphatic anhydride is 1.3-3 times of the amount of substance of hydroxyl in the monomer. In the acetylation reaction process, by-product fatty acid is removed, and the amount of substance of aliphatic anhydride is strictly controlled, so that the acetylation reaction is more complete, blockage caused by monomer volatilization is reduced, the prepared thermotropic liquid crystal polyester does not turn black and does notbubble in the reflow soldering process, and the mechanical property is excellent.
Owner:JIANGMEN DENGZHONGTAI ENG PLASTICS CO LTD
Additive for anisotropic conductive adhesives and preparation method thereof
InactiveCN103184017AUniform stabilityEquilibrium curing temperatureNon-macromolecular adhesive additivesMacromolecular adhesive additivesPolymer scienceGlycidyl methacrylate
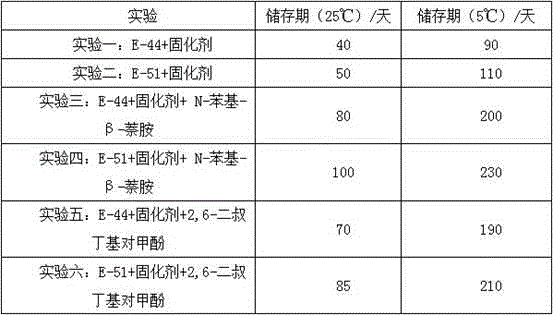
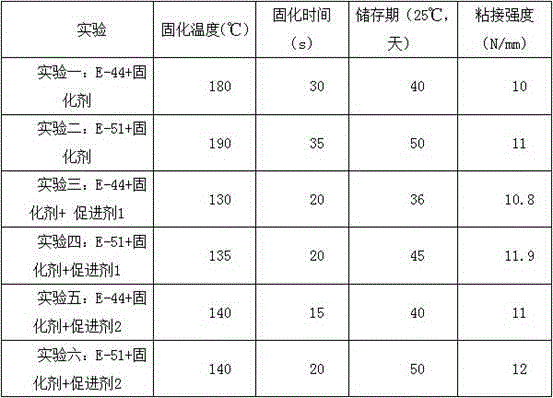
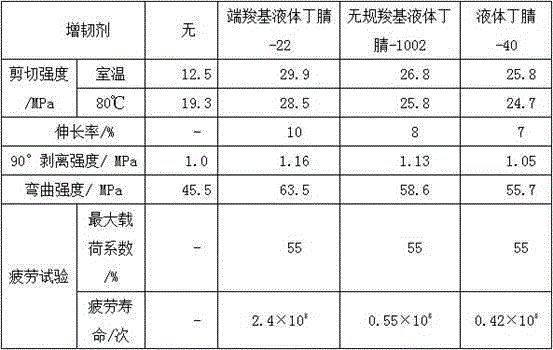
The invention discloses an additive for anisotropic conductive adhesives and a preparation method thereof. The additive consists of the following ingredients: microencapsulated long-chain imidazole derivatives, a stabilizer, a curing promoter, a plasticizer, a diluent and a coupling agent, wherein the stabilizer is N-phenyl-beta-naphthylamine or 2,6-di-tert-butyl-p-cresol, the curing promoter is a tri(-2ethylhexoic acid) salt of DMP-30 or an acetylacetone clathrate of manganese, the plasticizer is terminal carboxyl liquid butyronitrile-22, random carboxyl liquid butyronitrile-1002 or liquid butyronitrile-40, and the diluent is butyl glycidyl ether, phenyl glycidyl ether, glycidyl methacrylate, polyethylene glycol diglycidyl ether or polypropylene glycol diglycidyl ether. The additive for anisotropic conductive adhesives can ensure that each performance of an anisotropic conductive adhesive can reach a balanced and better level.
Owner:GUANGDONG DANBOND TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
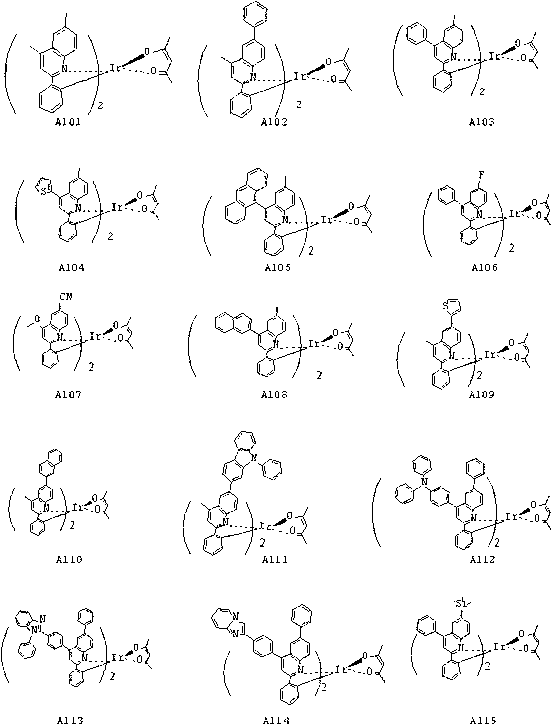
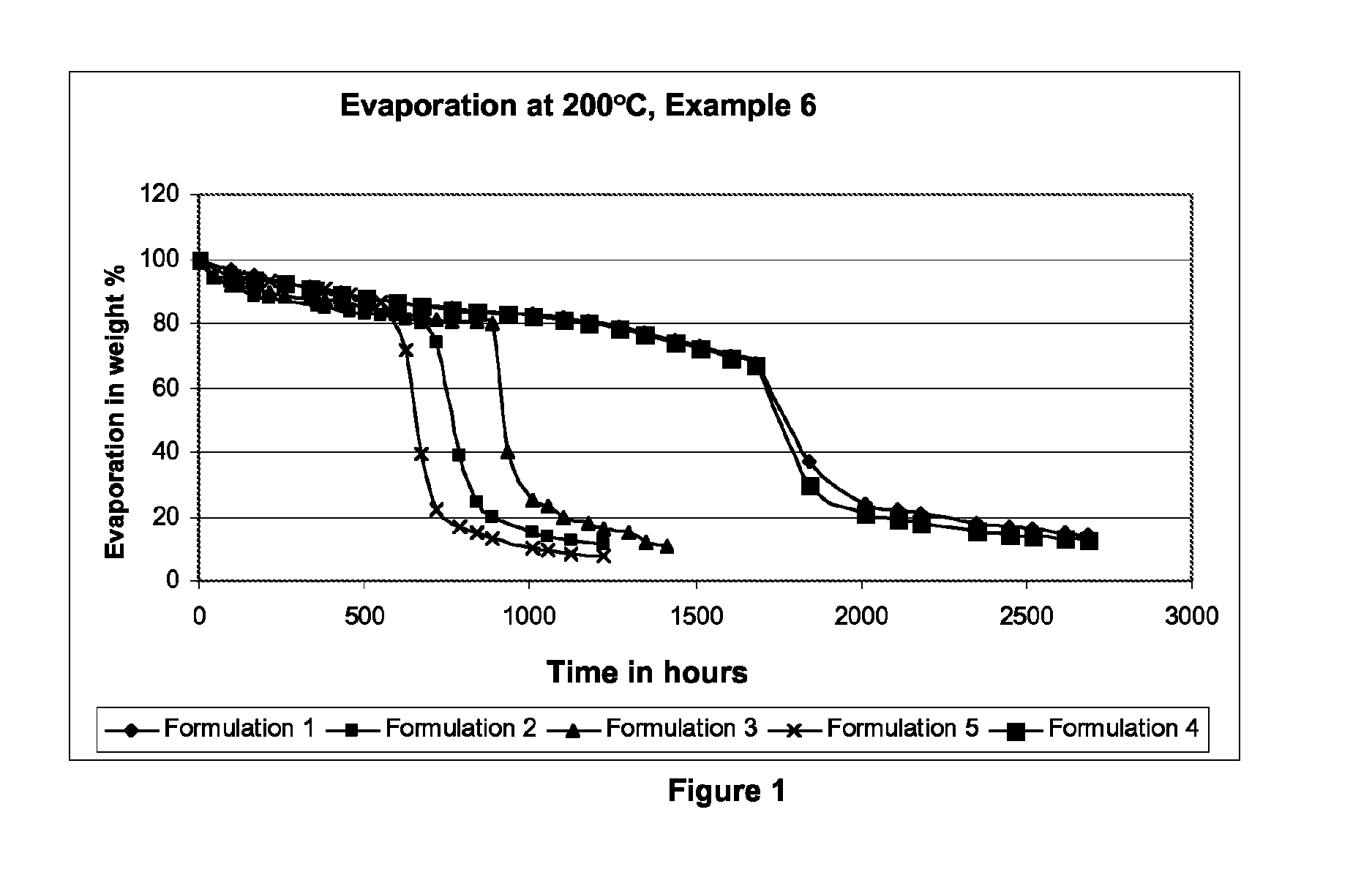
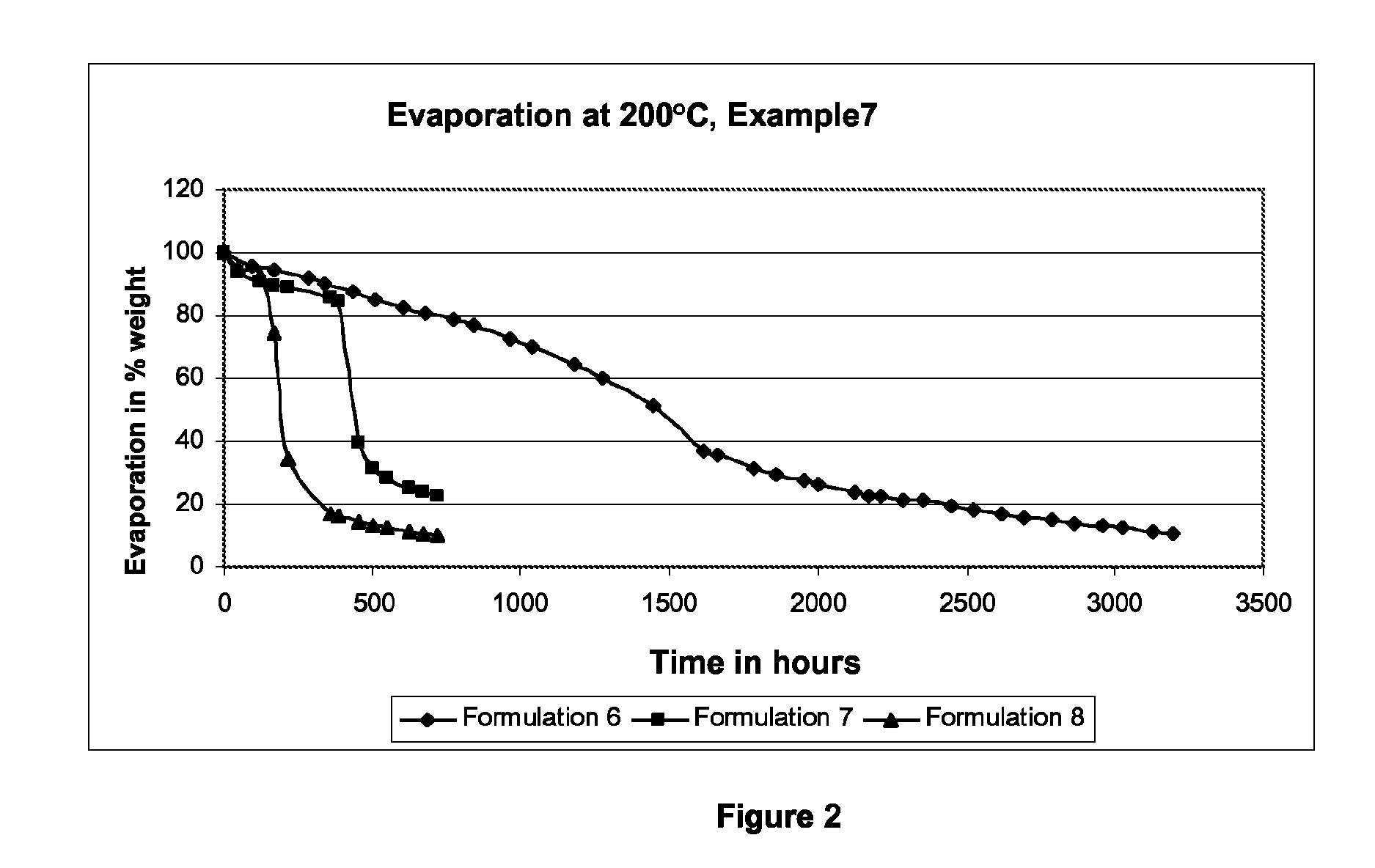
![Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices](https://images-eureka.patsnap.com/patent_img/f5ff0d6e-7f6b-4f87-98e8-ecec86345c4a/US08697890-20140415-D00000.png)
![Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices](https://images-eureka.patsnap.com/patent_img/f5ff0d6e-7f6b-4f87-98e8-ecec86345c4a/US08697890-20140415-D00001.png)
![Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices Dichroic-photochromic 2H-naphtho[1,2-b]pyran compounds and devices](https://images-eureka.patsnap.com/patent_img/f5ff0d6e-7f6b-4f87-98e8-ecec86345c4a/US08697890-20140415-C00001.png)