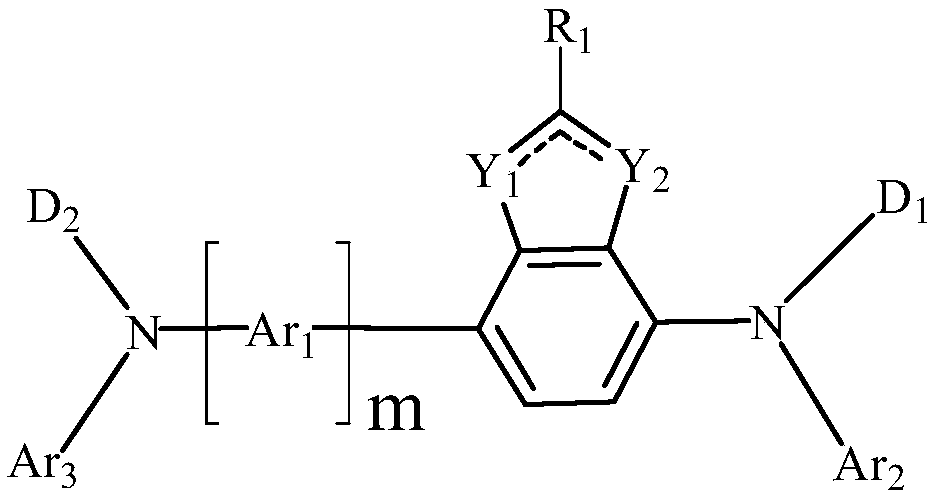
Benzoheterocyclic compound, display panel and display device
A heterocyclic compound and display panel technology, applied in organic chemistry, chemical instruments and methods, organic semiconductor devices, etc., can solve the problems of high light extraction efficiency, insufficient light extraction effect, and large refractive index difference, etc., to achieve high light extraction Efficiency, avoiding the effect of reducing color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Synthesis of Compound P1
[0096]
[0097] Weigh S1 (10mmol) into a 100mL two-necked flask, add 30mL of toluene degassed with nitrogen to dissolve S1, one port of the two-necked flask is connected to a constant pressure dropping funnel, and replace the gas in the reaction system with nitrogen. Weigh NBS (N-bromosuccinimide) (10.5mmol), add 20mL of toluene to dissolve it, and add the toluene solution of NBS to S1 After stirring for 2 h, the temperature of the reaction system was slowly raised to room temperature and stirred overnight. After the reaction was completed, 50 mL of deionized water was added to quench the reaction, extracted with dichloromethane (100 mL×3), and the organic phase was collected and washed with anhydrous Na 2 SO 4 The organic phase is dried. The dried organic phase was filtered, and the solvent was distilled off under reduced pressure with a rotary evaporator to obtain a crude product. The crude product was purified by gradient elution of ...
Embodiment 2
[0113] Synthesis of Compound P4
[0114]
[0115] Put S2 (10mmol), S8 (10.5mmol), tris(dibenzylideneacetone) dipalladium (0) (0.05mmol), sodium tert-butoxide (14mmol), tert-butylphosphine (0.2mmol) into a 50mL three-necked flask While stirring, degassing and nitrogen replacement were repeated three times quickly, and 20 mL of toluene was added through a syringe. The mixture was heated under reflux for 3 hours under nitrogen flow, and then left to cool to room temperature. Water was added to the cooled reaction solution, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was removed by distillation, and purified using column chromatography to obtain intermediate S9 (7.2 mmol, 72%).
[0116] MALDI-TOF MS: C30 h 22 N 2 S 4 , calculated m / z: 538.1; tested: 538.4.
[0117] Elemental Analysis Calculated: C, 66.88; H, 4.12; N, 5.20; S, 23.81; Tested: C, 66.90; H, 4.15; N, 5.18;
[0118] ...
Embodiment 3
[0127] Synthesis of Compound P5
[0128]
[0129] Under nitrogen protection, weigh compound S5 (25mmol), S12 (25mmol), [Pd 2 (dba) 3 ]·CHCl 3 (0.5mmol) and HP(tBu) 3 ·BF 4 (1.0mmol), was added to a 250mL two-necked flask. Inject 100mL of toluene into the two-necked flask (introduce N in advance 2 15min to remove oxygen), and then add 12mL of K with a concentration of 1M dropwise 2 CO 3 Aqueous solution (into N in advance 2 15min to remove oxygen), and stirred overnight at room temperature. After the reaction was completed, 100 mL of deionized water was added, followed by a few drops of 2M HCl. Extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 The organic phase is dried. The dried organic phase was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel column chromatography to obtain solid S13 (18.8 mmol, 75%).
[0130] MALDI-TOF MS: C 14 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com