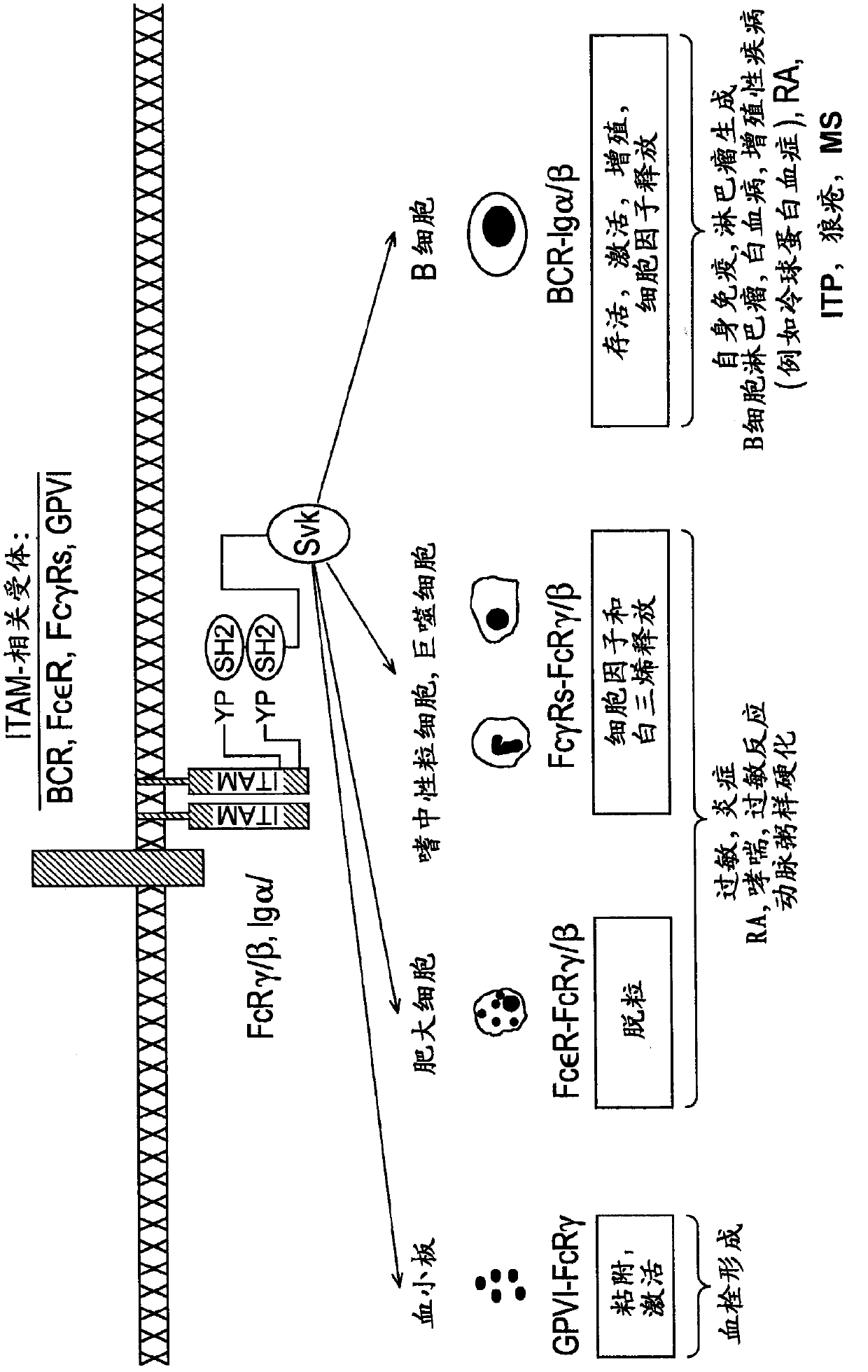
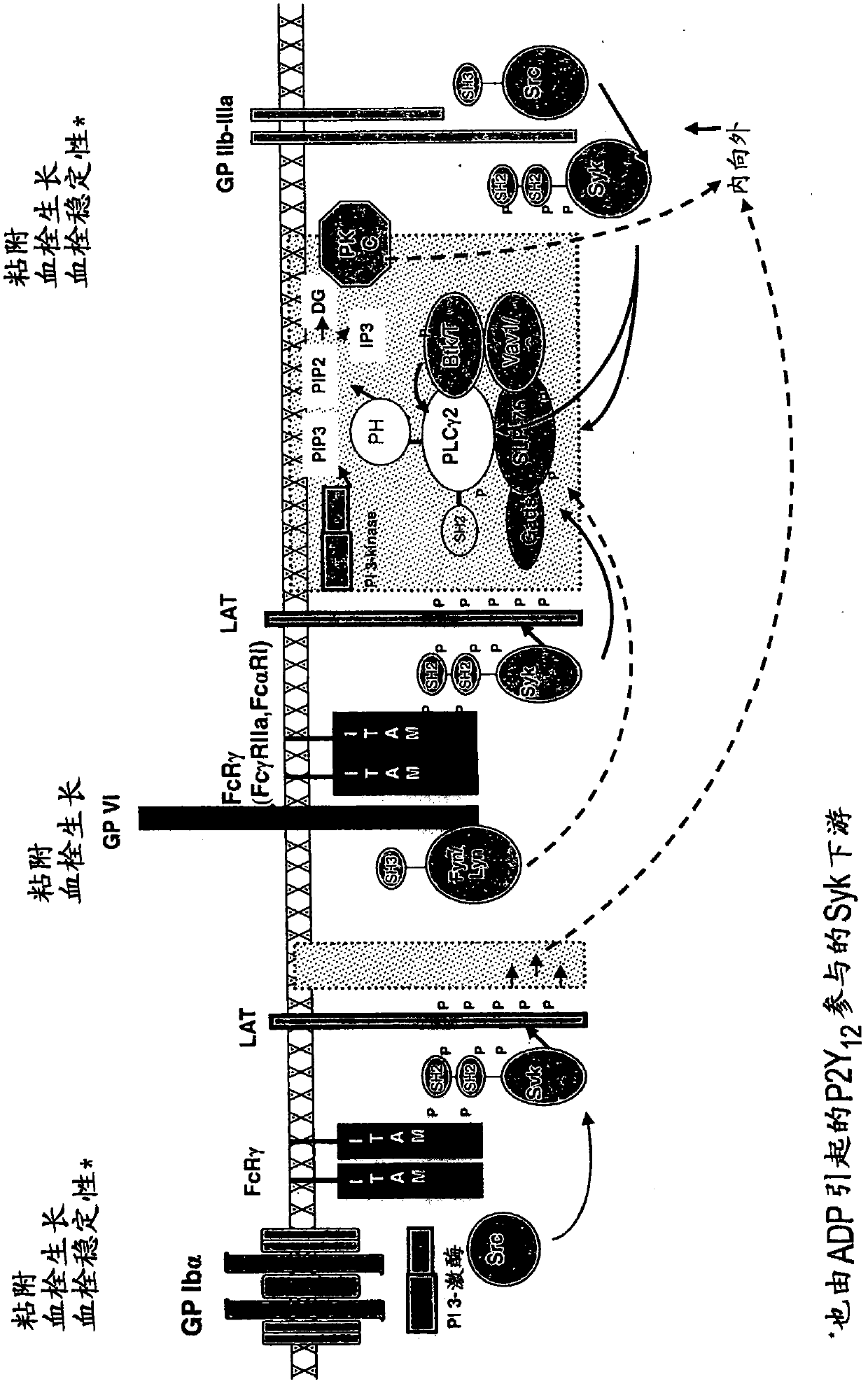
2, 6-diamino- pyrimidin- 5-yl-carboxamides as SYK or JAK kinases inhibitors
A compound, amino technology, applied in the preparation of the compound described in this article, the treatment of the disease field characterized by other indications, can solve problems such as Src kinase inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
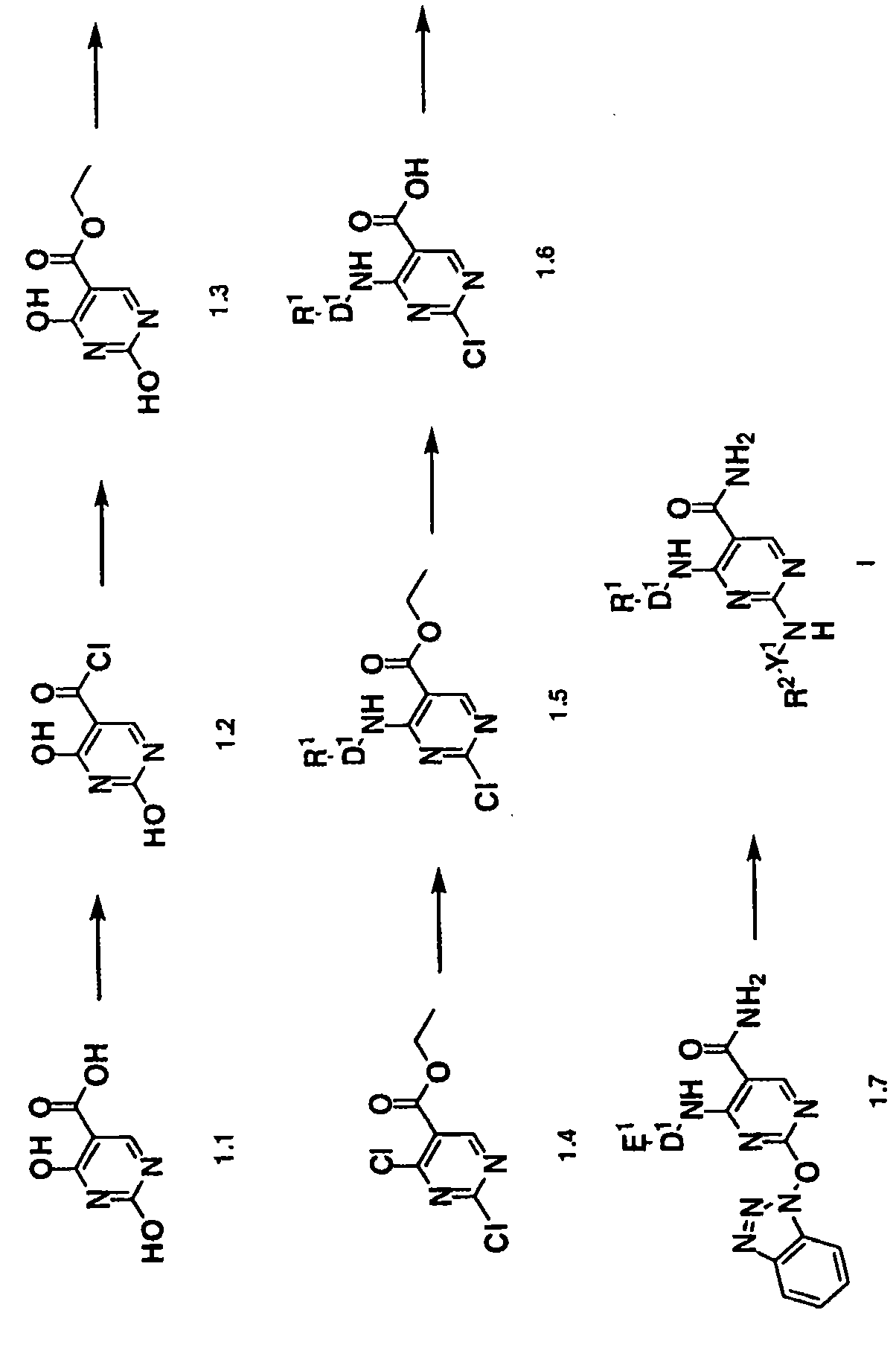
[0309] a. Compound
[0310] In one group of embodiments, the present invention provides a compound having formula (I), a tautomer or a pharmaceutically acceptable salt thereof:
[0311]
[0312] in:
[0313] Y 1 selected from:
[0314]
[0315] Z is O or S;
[0316] D. 1 selected from:
[0317] (a) By group R 5 Substituted phenyl, wherein phenyl is also optionally substituted by 1-2 substituents R 7a replace, R 7a Independently selected from C 1-8 Alkyl, C 1-8 Alkoxy, halo, C 1-8 Alkylsulfonyl and heterocyclyl;
[0318] R 5 selected from:
[0319] (i) heteroaryl;
[0320] (ii) heterocyclyl;
[0321] (iii)C 1-8 Alkylheterocyclyl;
[0322] (iv) phenylene heteroaryl
[0323] (v) phenylene heterocyclyl
[0324] (vi)-L-phenyl;
[0325] (vii)-L-heterocyclyl; and
[0326] (viii) acyloxy;
[0327] L is selected from -CO-, -O-, -SO 2 -, -CONH- and -CONHCH 2 -;
[0328] Each R 5 is optionally further substituted with 1-2 substituents independently selected ...
Embodiment 1
[0640] Example 1: 4-(4-(4-acetylpiperazin-1-yl)anilino)-2-((1R,2S)-2-aminocyclohexylamino)pyrimidine-5-carboxamide
[0641]
[0642] Step 1: To a solution of carboxylic acid 72.1 (85 g, 540 mmol) in thionyl chloride (425 mL) was slowly added pyridine (8.5 mL, 0.11 mmol) with stirring. The reaction was stirred overnight at 75 °C, then concentrated and dried in vacuo to a pale yellow powder. The yellow solid was slowly diluted with 750 mL of ethanol and refluxed overnight. The next day, the reaction was determined to be complete by HPLC, then cooled in an ice bath, and the solid was filtered and washed with ether to afford the ethyl ester 72.2 as an off-white powder (91 g, 87% for two steps). C 7 h 8 N 2 o 4 The MS measured value is (M+H) + 185.0.
[0643] Step 2: Ester 72.2 (22 g, 120 mmol) was dissolved in phosphorus oxychloride (60 mL, 600 mmol), the mixture was treated with N,N-diethylaniline (27 mL, 167 mmol), the mixture was heated to 105 °C until the reaction wa...
Embodiment 2
[0648] Example 2: 2-((1R,2S)-2-aminocyclohexylamino)-4-(4-(1,1-dioxo)thiomorpholineanilino)pyrimidine-5-carboxamide
[0649]
[0650] Using the same synthetic scheme shown in Example 1, substituting 4-(1,1-dioxo)thiomorpholine aniline for aniline 72.4, the above racemic compound was prepared. C 21 h 29 N 7 o 3 The MS measured value of S is (M+H) + 460.2. UVλ=236, 312nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com