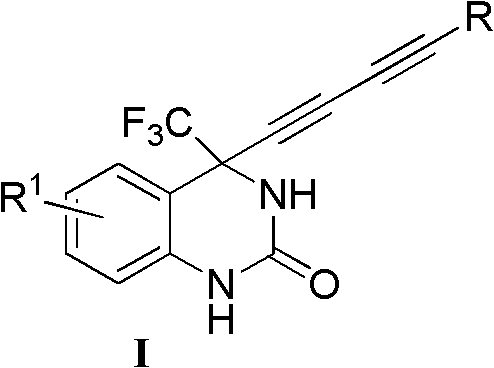
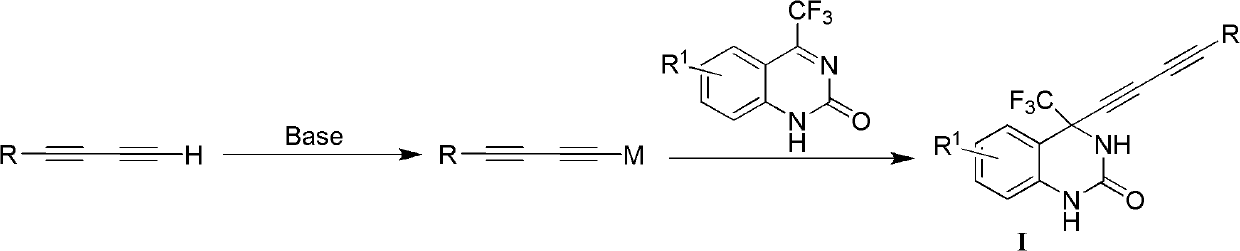
4-(substituted-1,3-diyne)-4-(trifluoromethyl)-3,4-dihydro substituted quinazoline-2-ketone compound as well as preparation method and application thereof
A technology for trifluoromethylquinazoline and ketone compounds, which is applied in 3 fields and achieves the effects of simple and feasible synthesis method, high yield and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Example 1: 6-Chloro-4-(penta-1,3-diynyl)-4-trifluoromethyl-3,4-dihydroquinazolin-2(1H)-one (1)
[0018]
[0019] At 0°C, 1.2M methylzinc-n-hexane solution (2.6mL, 3.0mmol) was added dropwise into a toluene solution (5mL) dissolved in 1,3-pentadiyne (192mg, 3.0mmol) for 10 minutes. After that, stir at the same temperature for 30 minutes. The reactant was then cooled to -15°C, a toluene solution (7 mL) dissolved with 6-chloro-4-trifluoromethylquinazolin-2(1H)-one (248.6 mg, 1.0 mmol) was added, and the reaction mixture Stir at 0°C for 24 hours. Cool to -10°C, add 3N HCl to quench the reaction and adjust the pH to 7, add 25 mL of ethyl acetate to the mixture, transfer the mixture to a separatory funnel, separate the organic layer, and wash with aqueous sodium chloride, The organic layer was dried by adding anhydrous sodium sulfate. After filtration, the solvent was distilled off, and rapid silica gel column separation was performed to obtain the target compound 1 (28...
example 2
[0020] Example 2: 6-fluoro-4-(penta-1,3-diynyl)-4-trifluoromethyl-3,4-dihydroquinazolin-2(1H)-one (2)
[0021]
[0022] At -78°C, 1.6M butyllithium n-hexane solution (0.6mL, 1.0mmol) was added dropwise into a tetrahydrofuran solution (10mL) dissolved in 1,3-decadiyne (134.2mg, 1.0mmol), 10 After 1 minute, the mixture was stirred at the same temperature for 30 minutes. Then the reactant was raised to -15°C, a tetrahydrofuran solution (6 mL) dissolved with 6-fluoro-4-trifluoromethylquinazolin-2(1H)-one (232.1 mg, 1.0 mmol) was added, and the reaction The mixture was stirred at 0°C for 24 hours and at room temperature for 6 hours. Cool to -10 ° C, add 3N HCl to quench the reaction and adjust the pH to 7, add 20 mL of ethyl acetate to the mixture, transfer the mixture to a separatory funnel, separate the organic layer, and then wash with aqueous sodium chloride, organic The layer was dried by adding anhydrous sodium sulfate. After filtration, the solvent was distilled off, a...
example 3
[0023] Example 3: 6-Chloro-4-(cyclopropan-1,3-diynyl)-4-trifluoromethyl-3,4-dihydroquinazolin-2(1H)-one (3)
[0024]
[0025] At 0°C, 1.5M ethylmagnesium bromide in ether (1.4mL, 2.0mmol) was added dropwise to cyclopropane-1,3-diyne (180.2mg, 2.0mmol) in ether (10mL) In 8 minutes, the addition was completed, and stirred at the same temperature for 30 minutes. The reactant was then cooled to -10°C, and a solution of ether (5 mL) dissolved with 6-chloro-4-trifluoromethylquinazolin-2(1H)-one (248.6 mg, 1.0 mmol) was added, and the reaction mixture Stir at 0°C for 24 hours. Cool to -10°C, add 3N HCl to quench the reaction and adjust the pH to 7, add 25 mL of ethyl acetate to the mixture, transfer the mixture to a separatory funnel, separate the organic layer, and wash with aqueous sodium chloride, The organic layer was dried by adding anhydrous sodium sulfate. After filtration, the solvent was distilled off, and rapid silica gel column separation was performed to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com