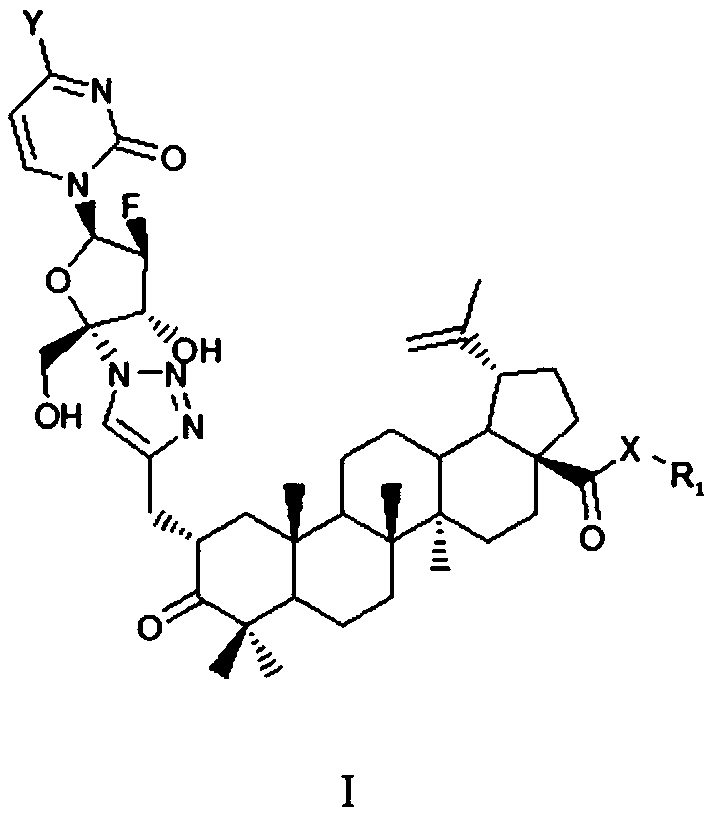
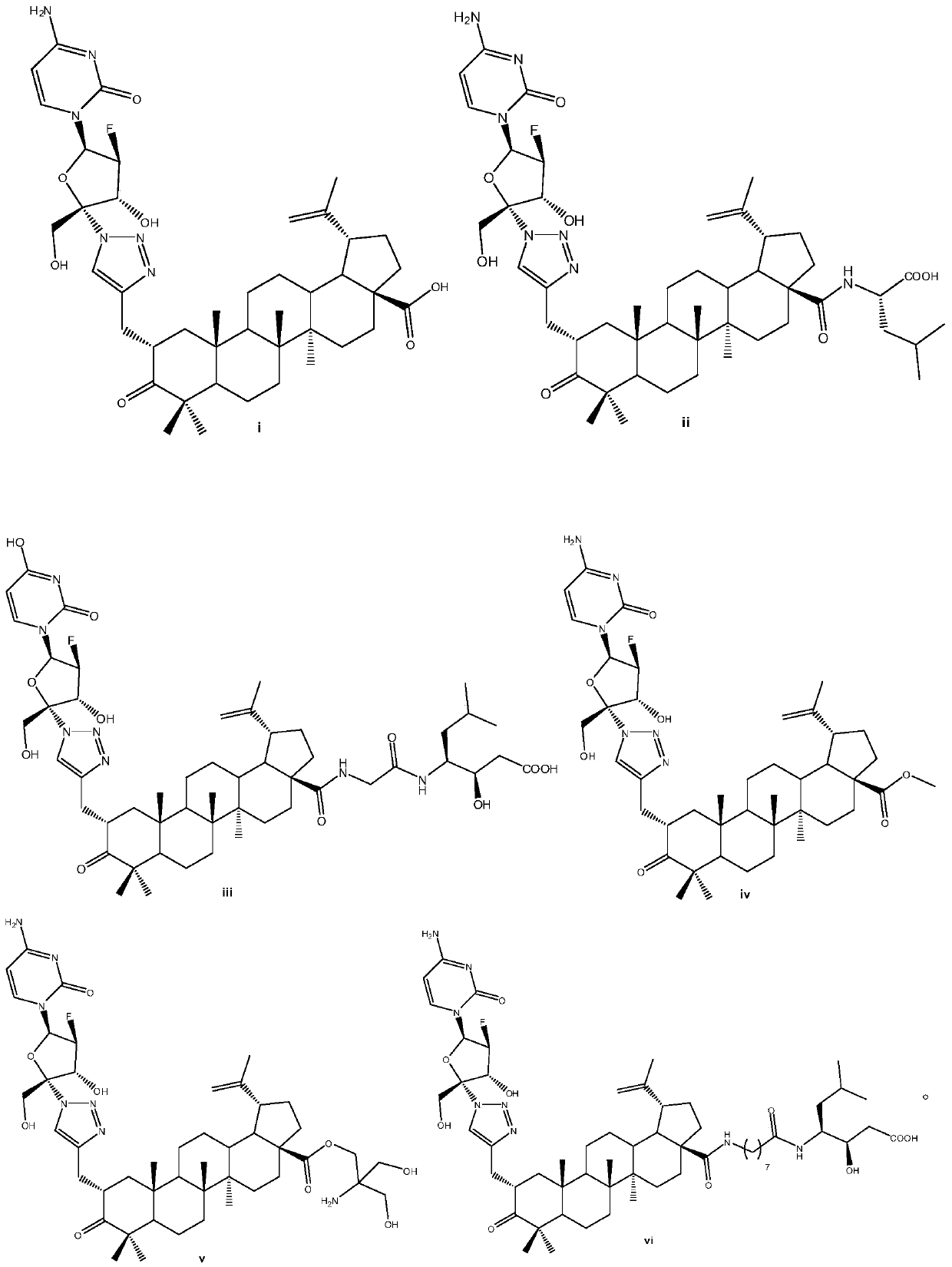
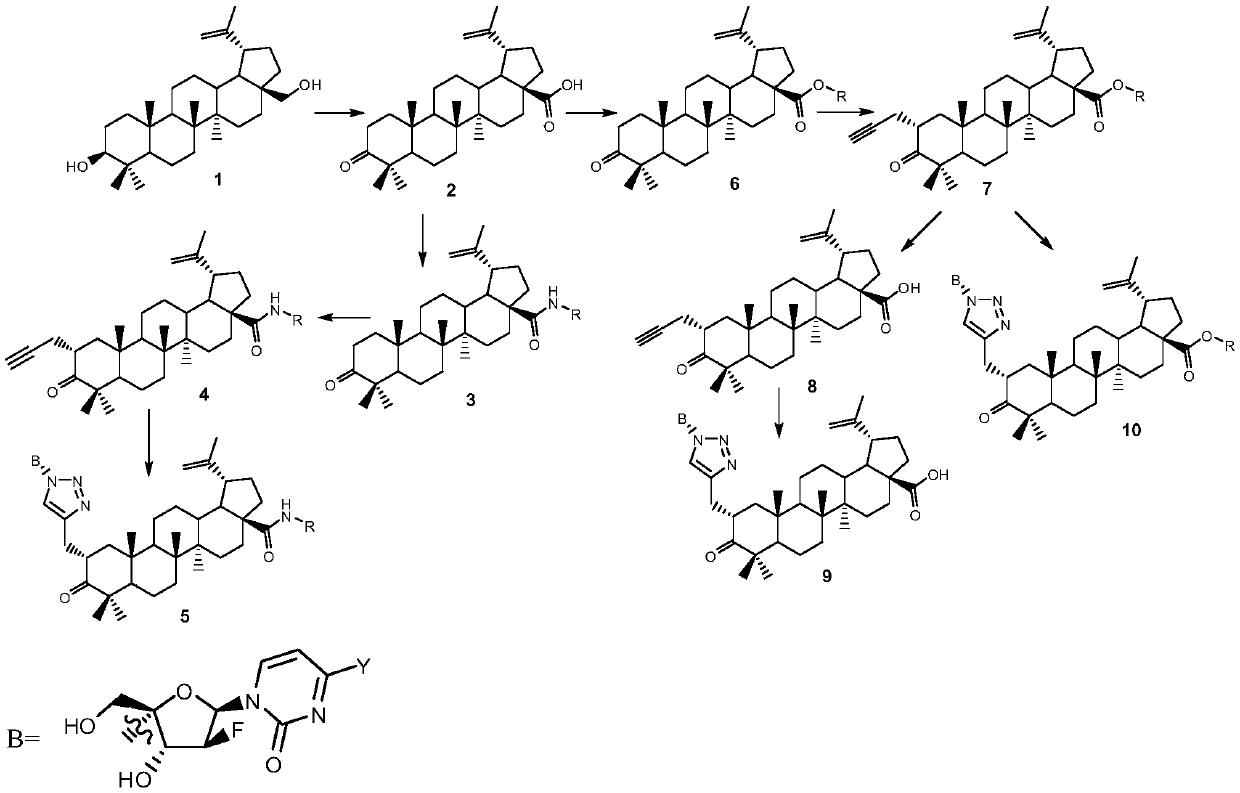
Betulin keto acid derivatives and their synthesis method and application
A technology of betulinic acid and betulinic acid, which is applied in the fields of medicine and its preparation and application, can solve the problems of low bioavailability in vivo transmission, metabolism, poor solubility, etc., achieve good application value, make up for poor water solubility and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of embodiment 1 compound iv
[0032] The synthesis path is as follows:
[0033]
[0034] in
[0035] Synthesis of Compound 2: Add betulin (20.0 g, 45.2 mmol) and acetone (400 mL) into a 1000 mL three-necked flask, then add the newly prepared Jones reagent (100 mL) dropwise under ice bath, and continue the reaction for 30 Minutes later, remove the ice bath, stir at room temperature for 8 hours, add methanol (250mL) and water (250mL) to terminate the reaction, evaporate the solvent, add water, extract with ethyl acetate, combine the organic phases, dry and concentrate, and separate by column chromatography to obtain a white solid 2 (12.2 g, 26.8 mmol, 59.3%). 1 HNMR (DMSO-d 6 ,400MHz) δ: 12.08(s,1H),4.70(s,1H),4.57(s,1H),2.90~2.99(m,1H),2.46~2.30(m,2H),2.25(dt,J= 12.7, 3.5Hz, 1H), 2.15~2.08(m, 1H), 1.87~1.73(m, 3H), 1.65(s, 3H), 1.62(s, 1H), 1.54(t, J=11.3Hz, 1H ),1.48~1.00(m,15H),0.98(s,3H),0.95(s,3H),0.93(s,3H),0.90(s,3H),0.85(s,3H).
[0036] Sy...
Embodiment 2
[0039] The synthesis of embodiment 2 compound ii
[0040] The synthesis path is as follows:
[0041]
[0042] Synthesis of Compound 2: Add betulin (20.0g, 45.2mmol) and acetone (400mL) to a 1000mL three-neck flask, then add the newly prepared Jones reagent (100mL) dropwise under ice bath, and continue the reaction for 30 Minutes later, remove the ice bath, stir at room temperature for 8 h, add methanol (250 mL) and water (250 mL) to terminate the reaction, evaporate the solvent to dryness, add water, extract with ethyl acetate, combine the organic phases, dry and concentrate, and separate by column chromatography to obtain a white solid 2 (12.2 g, 26.8 mmol, 59.3%). 1 HNMR (DMSO-d 6 ,400MHz) δ: 12.08(s,1H),4.70(s,1H),4.57(s,1H),2.90~2.99(m,1H),2.46~2.30(m,2H),2.25(dt,J= 12.7, 3.5Hz, 1H), 2.15~2.08(m, 1H), 1.87~1.73(m, 3H), 1.65(s, 3H), 1.62(s, 1H), 1.54(t, J=11.3Hz, 1H ),1.48~1.00(m,15H),0.98(s,3H),0.95(s,3H),0.93(s,3H),0.90(s,3H),0.85(s,3H).
[0043] Synthesis of Comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com