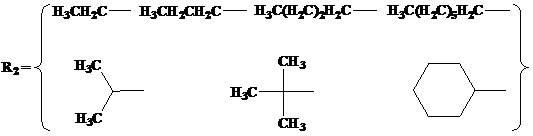
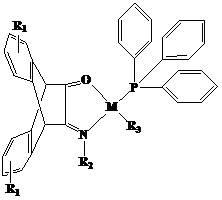
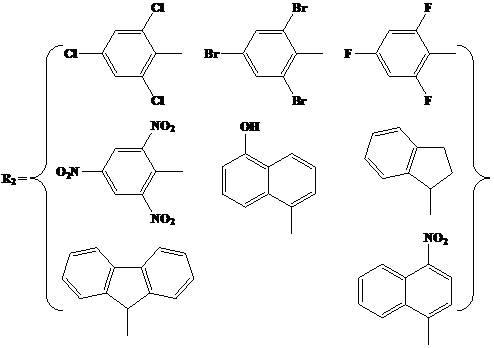
N,O-single ligand metal catalyst with stereochemical structure and preparation method thereof
A metal catalyst and three-dimensional structure technology, applied in the direction of organic chemistry, etc., can solve the problems of poor tolerance and low activity of polar monomers, and achieve the effects of low dielectric constant, high light transmittance, and good thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (I) Preparation of α-ketophenylimine ligands with three-dimensional structure.
[0041] Add 0.0143 mol of 9,10-dihydro-9,10-ethylene anthracene-11,12-dione, 0.0143 mol of aniline, and a catalytic amount of p-toluene into the reaction flask equipped with a reflux condenser and a water separator Sulfonic acid and 150 mL of toluene were used as solvents. After reacting for 2 hours, the novel stereoscopic α-ketobenimine ligand with a purity of more than 99% was obtained by recrystallization from n-hexane or distillation under reduced pressure.
[0042] (II) Preparation of mono(α-ketophenylimine)nickel(II) with three-dimensional structure of the main catalyst.
[0043] -78 o C slowly add the solution of n-butyllithium dissolved in toluene and n-hexane to the toluene containing the ligand prepared in step I, and the molar ratio of the two is 1:1. Then wait for the temperature of the mixture to slowly return to room temperature, and stir for 2 h. Then add 1 equivalent of t...
Embodiment 2
[0047] (I) Preparation of α-ketophenylimine ligands with three-dimensional structure.
[0048] With embodiment 1.
[0049] (II) Preparation of mono(α-ketophenylimine)palladium(II) complexes with the stereostructure of the main catalyst.
[0050] -78 o C slowly add the solution of n-butyllithium dissolved in toluene and n-hexane to the toluene containing the ligand prepared in step I, the molar ratio of the two is 1:1. Then wait for the temperature of the mixture to slowly return to room temperature, and stir for 2 h. Then add 1 equivalent of trans-[Pd(PPh 3 ) 2 (Ph)Cl]. Stir continuously overnight at room temperature, filter, and recrystallize to obtain a mono(α-ketophenylimine)palladium(II) complex with a new stereostructure.
[0051] (III) Catalytic polymerization of norbornene.
[0052] Heat the reaction bottle equipped with a magnetic stirrer and a branch with a heat gun, and simultaneously vacuumize it, fill it with nitrogen, and replace it three times to ensure th...
Embodiment 3
[0054] (I) Preparation of α-keto(2,6-dimethyl)phenylimine ligand with three-dimensional structure.
[0055] Add 0.0143 mol of 9,10-dihydro-9,10-ethylene anthracene-11,12-dione, 0.0143 mol of 2,6-dimethyl Aniline, a catalytic amount of p-toluenesulfonic acid and 200 mL of toluene were used as solvents. After reacting for 4 hours, recrystallize with n-hexane or distill under reduced pressure to obtain the α-keto(2,6-dimethyl)phenylimine ligand with a purity of more than 99%.
[0056] (II) Preparation of mono[α-keto(2,6-dimethyl)phenylimine]nickel(II) complex with stereostructure of procatalyst.
[0057] -78 o C slowly add the solution of n-butyllithium dissolved in toluene and n-hexane to the toluene containing the ligand prepared in step I, the molar ratio of the two is 1:1. Then wait for the temperature of the mixture to slowly return to room temperature, and stir for 2 h. Then add 1 equivalent of trans-[Ni(PPh 3 ) 2 (Ph)Cl]. Stir continuously overnight at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com