2,6-diphenyl naphthalene derivative and preparation method and application thereof
A technology of diphenylnaphthalene and its derivatives, which is applied in the field of 2,6-diphenylnaphthalene derivatives and its preparation, can solve the problems of increasing the material cost budget and increasing the manufacturing cost of OLED devices, and achieve the improvement of external quantum efficiency, Effect of improving transmittance and enhancing luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention also provides a preparation method of the 2,6-diphenylnaphthalene derivative, comprising:
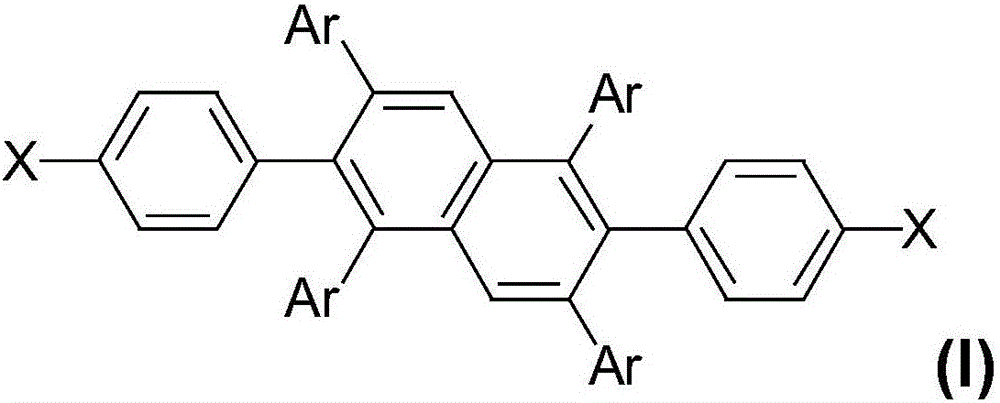
[0048] The compound shown in formula (A) and the compound shown in formula (B) are obtained the 2,6-diphenylnaphthalene derivative shown in formula (I) through coupling reaction under nitrogen protection:
[0049]
[0050] Among them, Ar is a C6-C60 aryl group, a C10-C60 fused aryl group, a C5-C60 six-membered heterocycle or a C4-C60 five-membered heterocycle, and X is a C6-C30 arylamino group or a C6-C30 of fused heterocycles.
[0051] According to the present invention, the intermediate shown in formula (A) is prepared according to the method shown below:
[0052] (1) 2,6-dichloronaphthalene shown in formula A-1 is reacted with elemental bromine to obtain a compound shown in formula A-2;
[0053] (2) reacting the compound represented by formula A-2 with Grignard reagent Ar-MgBr to obtain the intermediate represented by formula (A).
[0054]
[005...
Embodiment 1
[0063] Embodiment 1: the preparation of intermediate A
[0064]
[0065] (1) Synthesis of compound A-2: add 82ml fuming nitric acid (29%SO 3 ), followed by adding 11.0g (56mmol) of 2,6-dichloronaphthalene (compound A-1), 36g (225mmol) of Br 2 , 0.25g of Fe and 0.25g of I 2 , stirred vigorously at 60-70 °C for 6 hours. The mixed system was poured into ice water, filtered, and the solid was washed successively with aqueous sodium bisulfite, aqueous sodium bicarbonate and water. Recrystallization in toluene afforded 26.1 g (yield 91%) of compound A-2.
[0066] (2) Synthesis of intermediate A-a: Add 32ml of dry tetrahydrofuran and 5.8g (32mmol) of phenylmagnesium bromide successively to a dry 150ml three-necked flask, and dissolve 2.45g (4.0mmol) of compound A-2 in 20ml of dry in THF, in N 2 Added to the reaction system under the protection of , and stirred at room temperature for 12 hours. At 0°C, 6.35g (25mmol) of I 2 Added to the reaction system, stirred at room tempe...
Embodiment 2
[0070] Embodiment 2: the synthesis of compound TM1
[0071] Add 684mg (1mmol) of intermediate A-a and 231mg (0.2mmol) of tetrakis (triphenylphosphine) palladium mixture in 10ml of deaerated toluene, under N 2 Under protection, the system was heated to 60°C and stirred for 5 minutes. 636 mg (2.2 mmol) of triphenylamine-4-boronic acid, 2.5 ml of deaerated ethanol and 5 ml of deaerated 2M sodium carbonate aqueous solution were sequentially added to the system, and stirred at 80° C. for 21 hours. The reaction system was poured into a large amount of water and extracted with dichloromethane. The organic layer was washed successively with saturated brine and water, dried over anhydrous magnesium sulfate, and distilled under reduced pressure. The residual solid was purified by column chromatography (hexane / dichloromethane=4:4, V / V), and recrystallized in a mixed solution of hexane / chloroform to obtain 597 mg (0.65 mmol) of compound TM1 with a yield of 65 %. Mass Spectrum m / z: 919...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous efficiency | aaaaa | aaaaa |
| Luminous efficiency | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com