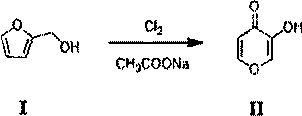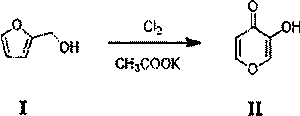Synthesis method of 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid
A synthesis method, benzyloxy technology, applied in the field of organic chemical synthesis, can solve the problems of harsh reaction conditions, difficulty in production scale-up, low process safety, etc., and achieve the effect of controllable conditions, strong repeatability and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Synthesis of 3-hydroxy-4H-pyran-4-one (compound II)
[0045]
[0046] Add methanol (60L), water (40L) and sodium acetate (20Kg) into the reaction kettle, and cool down to -5°C. Dissolve furfuryl alcohol (compound I) (40kg) in methanol (40L) and water (20L), and add it dropwise into the reaction kettle at a temperature controlled -5°C, and feed chlorine gas (57.8kg) into the reaction kettle while adding dropwise. Keep warm for 1h, the reaction is over, keep warm overnight, and filter. The mother liquor was heated to 50°C, stirred for 2h, and cooled to room temperature. filter. Concentrate under reduced pressure to remove methanol and crystallize to obtain 26.7 kg of 3-hydroxy-4H-pyran-4-one (compound II) with a purity of 95% and a yield of 59%. LC-MS: [M+H] + =113.02.
[0047] 2. Synthesis of 3-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one (compound III)
[0048]
[0049] 3-Hydroxy-4H-pyran-4-one (compound II) (26.7kg) and methanol (134L) were added to the reac...
Embodiment 2
[0057] 1. Synthesis of 3-hydroxy-4H-pyran-4-one (compound II)
[0058]
[0059] Add ethanol (30L), water (20L) and sodium acetate (10Kg) into the reaction kettle, and cool down to 5°C. Dissolve furfuryl alcohol (Compound I) (20kg) in ethanol (20L) and water (10L), and add it dropwise into the reaction kettle at a temperature of 5°C. Chlorine gas (25.5kg) is introduced into the reaction kettle while adding dropwise. Keep warm for 1h, the reaction is over, keep warm overnight, and filter. The temperature of the mother liquor was raised to 70°C, stirred for 2h, and cooled to room temperature. filter. Concentrate under reduced pressure to remove ethanol and crystallize to obtain 14.2 kg of 3-hydroxy-4H-pyran-4-one (compound II) with a purity of 94% and a yield of 62%. LC-MS: [M+H] + =113.02.
[0060] 2. Synthesis of 3-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one (compound III)
[0061]
[0062] 3-Hydroxy-4H-pyran-4-one (compound II) (14.2 kg) and methanol (70 L) were added...
Embodiment 3
[0070] 1. Synthesis of 3-hydroxy-4H-pyran-4-one (compound II)
[0071]
[0072] Add tetrahydrofuran (30L), water (20L) and potassium acetate (10kg) into the reaction kettle and cool down to 5°C. Dissolve furfuryl alcohol (compound I) (20kg) in tetrahydrofuran (20L) and water (10L), and add it dropwise into the reaction kettle at a temperature controlled 5°C, and feed chlorine gas (30kg) into the reaction kettle while adding dropwise. Keep warm for 1h, the reaction is over, keep warm overnight, and filter. The mother liquor was heated to 60°C, stirred for 2h, and cooled to room temperature. filter. Concentrate under reduced pressure to remove tetrahydrofuran and crystallize to obtain 12.4 kg of 3-hydroxy-4H-pyran-4-one (compound II) with a purity of 95% and a yield of 54%. LC-MS: [M+H] + =113.02.
[0073] 2. Synthesis of 3-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one (compound III)
[0074]
[0075] 3-Hydroxy-4H-pyran-4-one (compound II) (12.4 kg) and methanol (76 L) we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com