Mono-aryl thioether compound as well as preparation method and application thereof
A technology for aryl sulfides and compounds, which is applied in the field of monoaryl sulfide compounds and their preparation, and can solve problems such as harsh requirements, difficult raw materials, reaction conditions, and cumbersome operation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
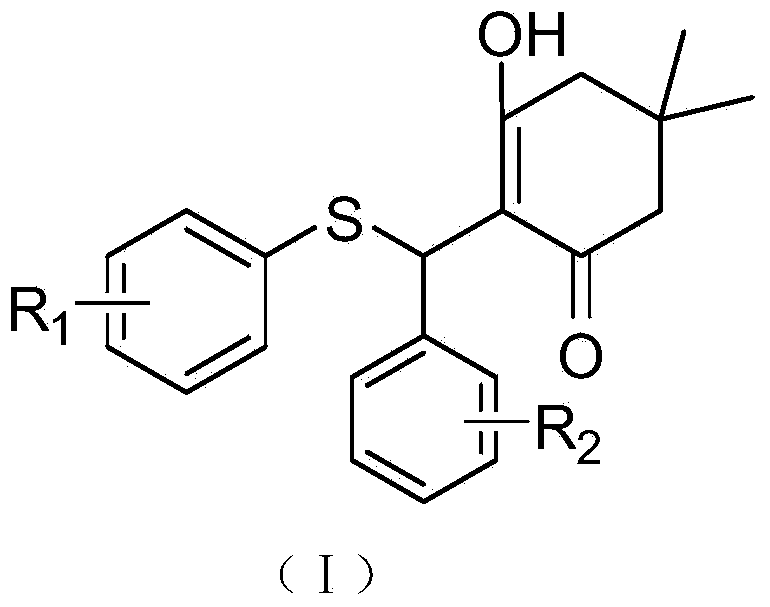
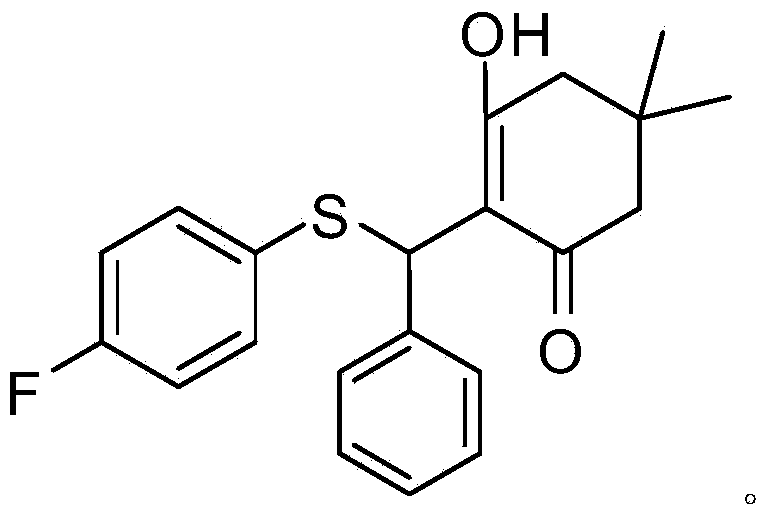
[0064] Compound A: Its chemical structural formula is shown in (I), where R 1 is 4-fluoro, R 2 is hydrogen; its chemical formula is: C 21 h 21 FO 2 S; chemical name: 2-((4-fluorophenylthio)(phenyl)methyl)-3-hydroxy-5,5-dimethylcyclohex-2-ene.
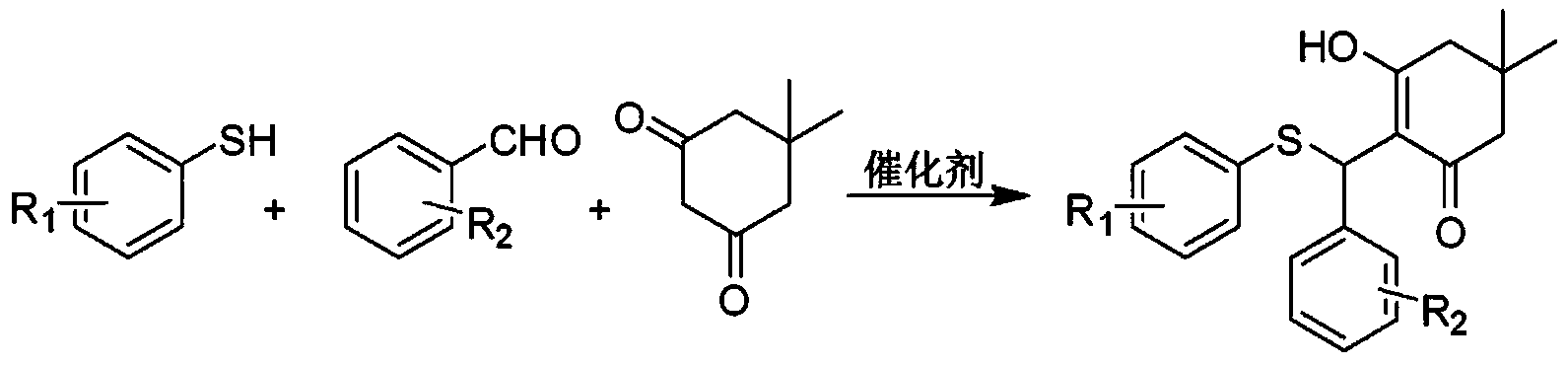
[0065] a) Mix 1 mmol of benzaldehyde, 145 mg of 4-fluorothiophenol, 140 mg of 5,5-dimethyl-1,3-cyclohexanedione and 38 mg of catalyst silica gel sulfuric acid, put them into a reaction vessel, and then Heating and stirring in an oil bath, using a TLC plate to follow the reaction process until the aromatic aldehyde is completely reacted to obtain a solution containing compound A precipitate;
[0066] b) Add dichloromethane to the above solution, stir to dissolve compound A precipitate, and then filter to obtain silica gel sulfuric acid filter cake and filtrate;
[0067] c) The filtrate was concentrated under reduced pressure, separated and purified through a 200-300 mesh silica gel column, and then rinsed with petroleum ether, and t...
Embodiment 2
[0078] Compound B: Its chemical structural formula is shown in (I), where R 1 is 2-fluoro, R 2 is hydrogen; its chemical formula is: C 21 h 21 FO 2 S; chemical name: 2-((2-fluorophenylthio)(phenyl)methyl)-3-hydroxy-5,5-dimethylcyclohex-2-ene.
[0079] a) Mix 1 mmol of benzaldehyde, 110 mg of 2-fluorothiophenol, 130 mg of 5,5-dimethyl-1,3-cyclohexanedione and 30 mg of catalyst dodecylbenzenesulfonic acid, and put them into a reaction vessel , and then heated and stirred in an oil bath at 85° C., using a TLC plate to track the reaction process until the aromatic aldehyde was completely reacted to obtain a solution containing compound B precipitate;
[0080] b) Add dichloromethane to the above solution, stir to dissolve compound B precipitate, and then filter to obtain dodecylbenzenesulfonic acid filter cake and filtrate;
[0081] c) Concentrate the filtrate under reduced pressure, pass through a 200-300 mesh silica gel column for separation and purification, and then use a ...
Embodiment 3
[0092] Compound C: Its chemical structural formula is shown in (I), where R 1 is 4-methoxy, R 2 is hydrogen; its chemical formula is: C 22 h 24 o 3 S; chemical name: 2-((4-methoxyphenylthio)(phenyl)methyl)-3-hydroxy-5,5-dimethylcyclohex-2-ene.
[0093] a) Mix 1 mmol of benzaldehyde, 125 mg of 4-methoxythiophenol, 135 mg of 5,5-dimethyl-1,3-cyclohexanedione and 40 mg of catalyst p-toluenesulfonic acid, and put them into a reaction vessel. Then heat and stir in an oil bath at 90°C, and use a TLC plate to track the reaction process until the aromatic aldehyde is completely reacted to obtain a solution containing compound C precipitate;
[0094] b) Add dichloromethane to the above solution, stir to dissolve the compound C precipitate, and then filter to obtain p-toluenesulfonic acid filter cake and filtrate;
[0095]c) Concentrate the filtrate under reduced pressure, pass through a 200-300 mesh silica gel column for separation and purification, and then use a mixture of petro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com