Pentachlorocyclopropane preparation method
A technology of pentachlorocyclopropane and trichloropropene, which is applied in the field of preparation of pentachlorocyclopropane, and can solve problems such as high tension of three-membered rings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
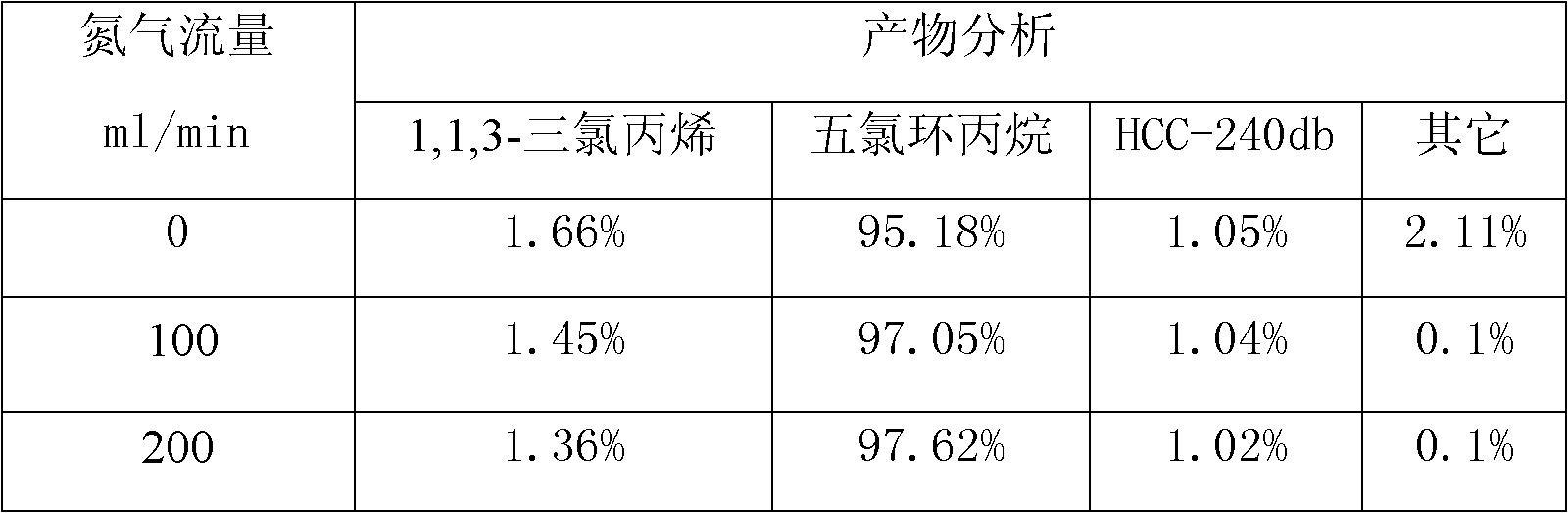
[0018] In a 1000ml three-neck flask, a reflux condenser is installed in the middle, chlorine gas is passed through one side, and a thermometer is installed on the other side, and 1000g of 1,1,3-trichloropropene, anhydrous FeCl 3 0.3 grams, chlorine gas flow rate 200ml / min, reaction time 12hr, the sample was treated with nitrogen gas for 20min before the reaction, without photocatalysis, the reaction results are shown in Table 1:
[0019] Table 1 Effect of nitrogen flow rate on the reaction
[0020]
Embodiment 2
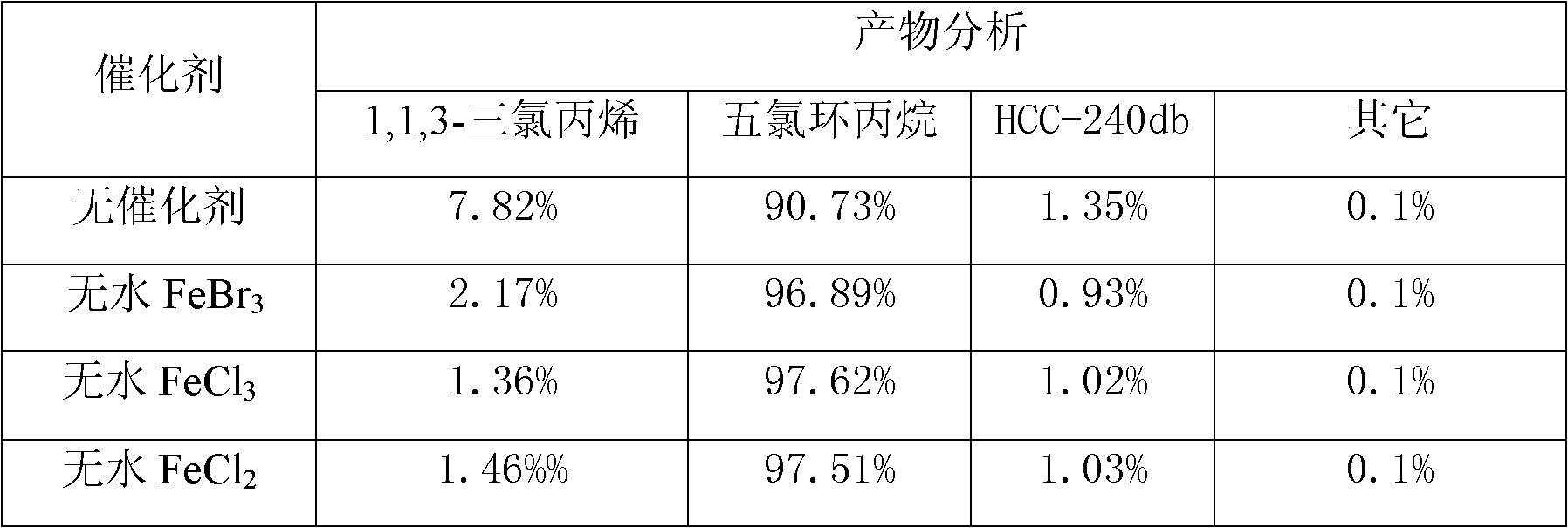
[0022] In a 1000ml three-neck flask, a reflux condenser is installed in the middle, chlorine gas is passed through one side, and a thermometer is installed on the other side, 1000g of 1,1,3-trichloropropene (water content 23ppm) is added, and anhydrous FeCl 3 , Anhydrous FeBr 3 , Anhydrous FeCl 2 0.3 grams each, chlorine gas flow rate 200ml / min, 200ml / min nitrogen gas treatment sample for 20min, reaction temperature 20±0.5℃, no photocatalysis, reaction time 12hr, reaction results are shown in Table 2:
[0023] Table 2 Effect of catalyst on reaction result
[0024]
Embodiment 3
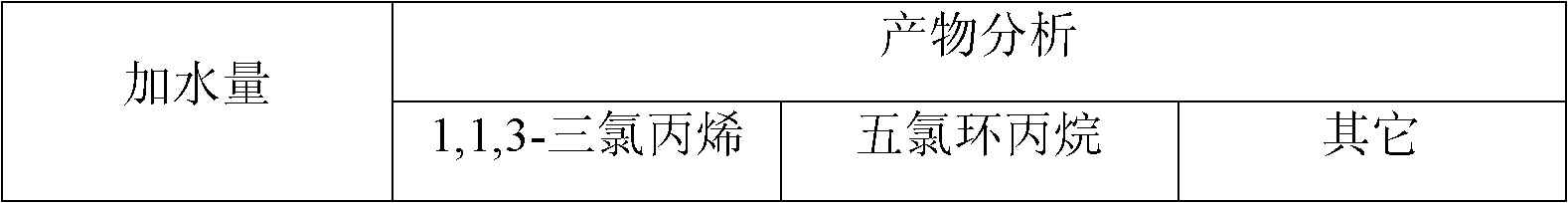
[0026] In a 1000ml three-neck flask, a reflux condenser is installed in the middle, chlorine gas is passed through one side, and a thermometer is installed on the other side, and 1000g of 1,1,3-trichloropropene, anhydrous FeCl 3 0.3 grams, chlorine gas flow rate 200ml / min, 200ml / min nitrogen gas treatment sample sample for 20min, reaction temperature 20±0.5℃, no photocatalysis, reaction time 12hr, reaction results are shown in Table 3:
[0027] Table 3 Effect of moisture on the reaction
[0028]
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com