Eriocalyxin A and Eriocalyxin B and preparation method thereof
A technology for calyx lactones and lactones, which is applied in the field of organic synthesis of natural products, can solve the problems such as no reports on the preparation methods of calyxolactone A and calyxolide B, and achieves a novel synthetic route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
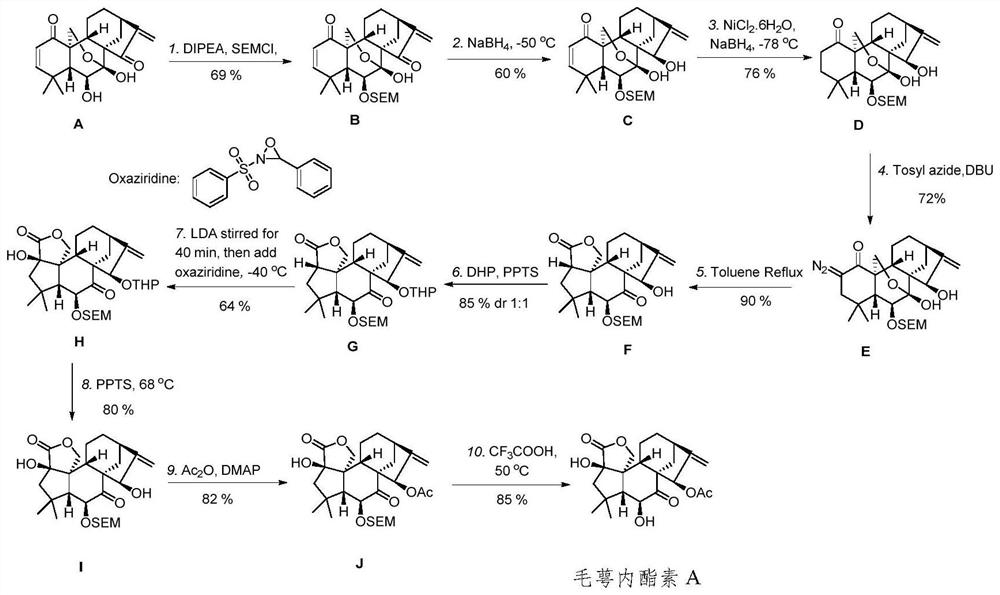
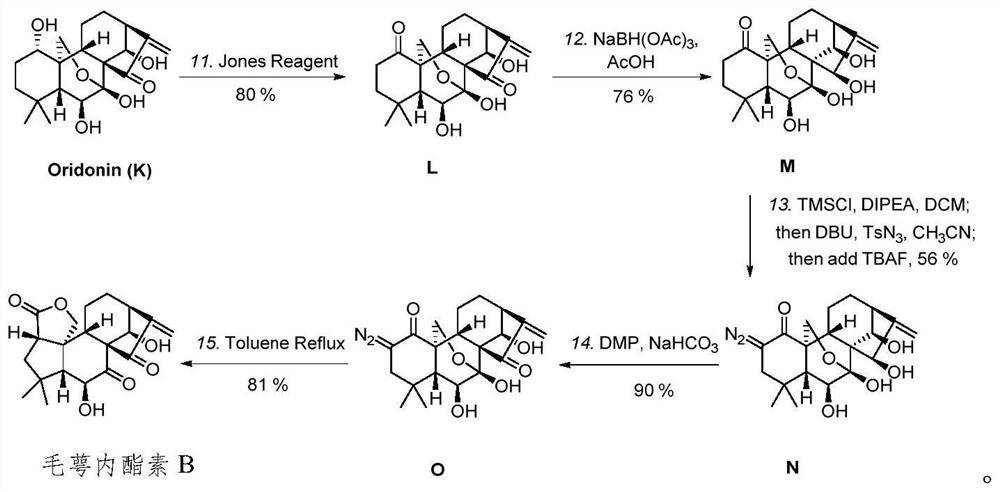
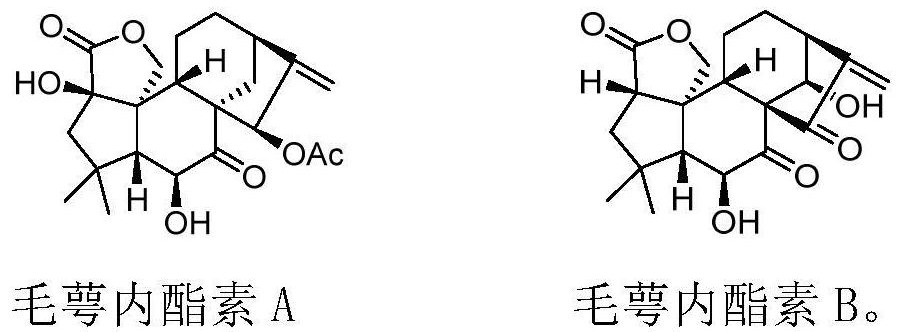
[0064] The novel enantio-kaurane diterpenoid compound prepared by the present invention is maoelactone A, which is isolated from medicinal plants, and is named maoelactone A in English. Taking calyxin A as the starting material, the chemical synthesis of calyxolactone A is realized by chemical reaction. Using oridonin as raw material, the structural modification precursor calyxolide B was constructed by Wolff rearrangement tandem lactonization reaction. The structural formula of the above compound is as follows:
[0065]
[0066] The preparation method of the novel enantio-kaurine diterpenoids tricolide A and the structure modification precursor tricolide B of the present invention is as follows:
[0067] (1) Preparation of Intermediate B: Compound A (0.01-100 g) was dissolved in dichloromethane (1-100 mL), and diisopropylethylamine (1-100 mL) was added. SEM-Cl (1-100 mL) was slowly added dropwise in an ice bath at 0°C. The reaction was then placed at room temperature fo...
Embodiment 2
[0098] (1) Preparation of Intermediate B: Compound A (20.0 g, 58 mmol, 1.0 equiv) was dissolved in 30 mL of dichloromethane, and diisopropylethylamine (11.5 mL, 87 mmol, 1.5 equiv) was added. SEM-Cl (15.0 mL, 87 mmol, 1.5 equiv) was slowly added dropwise under an ice bath at 0°C. The reaction was then placed at room temperature for 4 hours. After the completion of the reaction was detected by TLC, saturated NaHCO 3 (30 mL) quenched, extracted with EtOAc (3×300 mL), washed with saturated brine (50 mL), and anhydrous MgSO 4 After drying, filtration, and purification by column chromatography after concentration, eluent: petroleum ether / ethyl acetate=12:1, amorphous solid compound B was obtained. Data for B:R f = 0.41(silica, petroleum ether / acetone 4:1); (MeOH, c 0.07); 1 H NMR (600MHz, CDCl 3 )δ=6.71(d, J=10.1, 1H), 6.29(s, 1H), 5.86(d, J=10.1, 1H), 5.82(s, 1H), 5.25(d, J=1.4, 1H), 5.02 (d, J=7.1, 1H), 4.77 (d, J=7.1, 1H), 4.34 (dd, J=10.3, 1.3, 1H), 4.07 (ddd, J=12.6, ...
Embodiment 3
[0118] Cell-level antitumor activity test of the compounds of the present invention.
[0119] 1. Experimental purpose
[0120] The antitumor activity test of the compounds of the present invention was carried out, and the in vitro antitumor activity of the compounds was evaluated by measuring the growth inhibitory activity of the compounds on human tumor cells.
[0121] 2. Experimental materials
[0122] Human non-small cell lung cancer cell line A549, human liver cancer cell HepG2, human chronic myeloid leukemia cell K562 and human acute promyelocytic leukemia cell HL60 are all gifts from the research group of researcher Li Jia, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, human breast cancer cells MDA-MB-435 was purchased from the Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0123] 3. Test principle
[0124] Cell proliferation was detected by MTS colorimetry. MTS is a new analog of MTT, the full n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com