A kind of asymmetric cyclobutane derivative and its preparation method and application
A derivative and asymmetric technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of narrow substrate applicability, no existing, few successful cases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
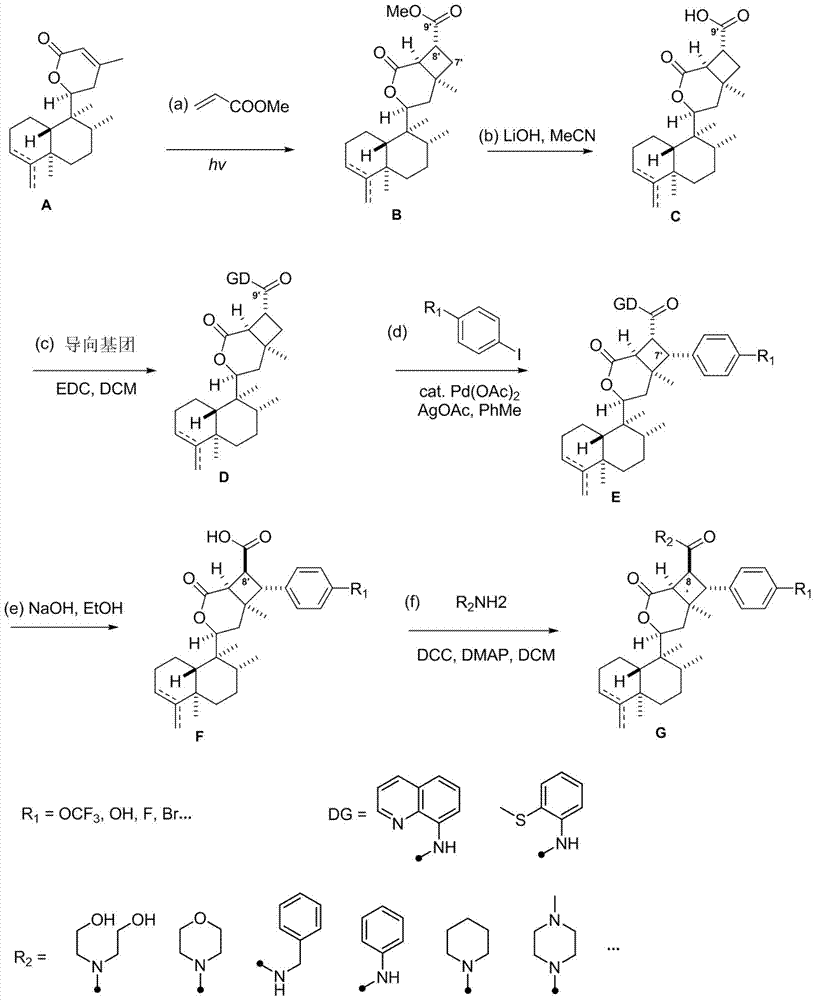
[0052] The asymmetric cyclobutane derivatives of the present invention are the synthetic derivatives G1-G8 of broom-shaped fragrant tea vegetable lactone compounds, the English name is scopariusicide derivatives G1-G8, and the compounds are obtained by separating from medicinal plants Heteroditerpene scopariusicides are a series of artificial analogues synthesized by six-step chemical reactions using the precursor compound ent-crodane diterpene A as the starting material. The basic structure is characterized by an asymmetric cyclobutene The different substituents on the side chains and aromatic rings of the enantio-crodane heteroditerpenes of the alkane fragments determine the diversity of this class of compounds. The structural formula of some compounds is:
[0053]
[0054] The preparation method of the asymmetric cyclobutane derivatives of the present invention: the method uses natural diterpene lactone A as the starting material, through intermolecular 2+2 photochemical...
Embodiment 2
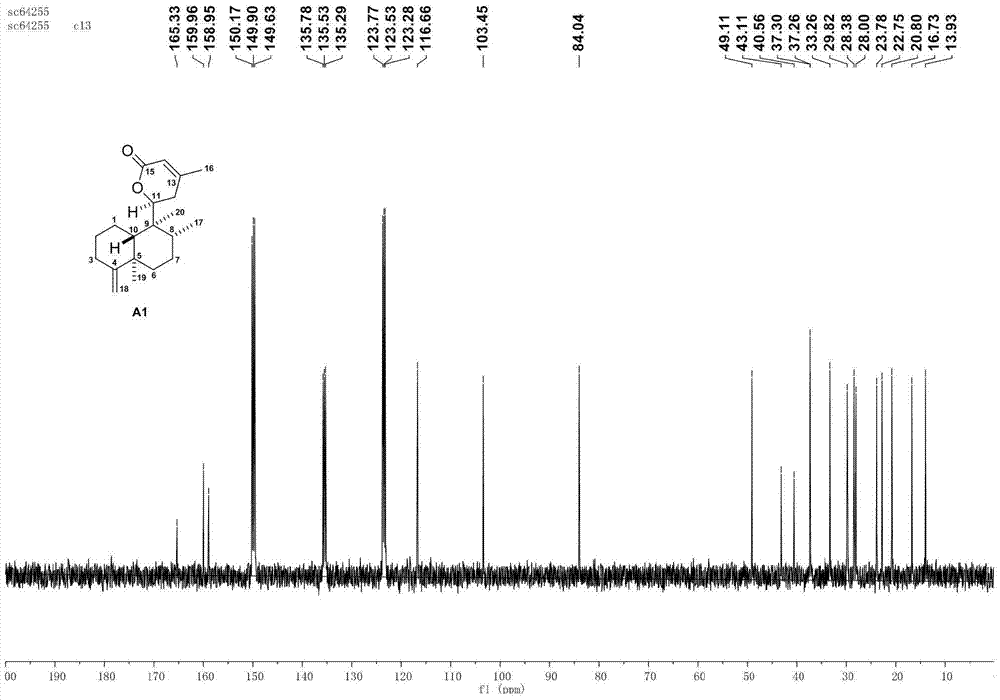
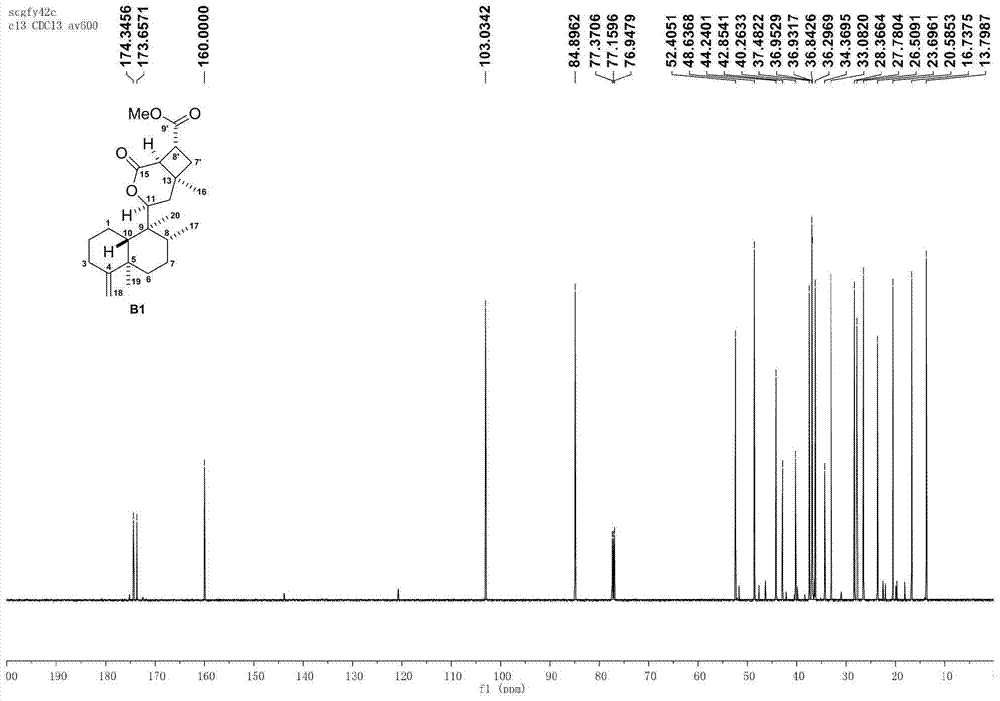
[0070] Using the natural diterpene lactone A1 isolated from the broom-like fragrant tea as the starting material, through the intermolecular 2+2 photochemical reaction (a), methyl ester alkali hydrolysis (b), and the upper guiding group (c) The intermediate D1 is obtained through three-step reaction, and then the aryl group (d) is activated by palladium-catalyzed carbon-hydrogen to obtain the synthetic derivatives G1-G2 of bristle-shaped catechin lactone compounds. The specific four-step chemical reaction is as follows:
[0071] .
[0072] (a) Preparation of intermediate B1 by intermolecular 2+2 photochemical reaction: Add precursor compound A (200mg per tube) to two 15mL quartz tubes, then add 10mL of analytically pure acetonitrile to dissolve the sample, and then add 1mL of methyl acrylate Esters were reacted in a photoreactor with a wavelength of about 300nm for 1 day. The sample was evaporated to dryness with a rotary evaporator, and various purification methods were us...
Embodiment 3
[0077] Using the natural diterpene lactone A1 isolated from the broom-like fragrant tea as the starting material, through the intermolecular 2+2 photochemical reaction (a), methyl ester alkali hydrolysis (b), and the upper guiding group (c) Three-step reaction to obtain intermediate D1, and then through palladium-catalyzed carbon-hydrogen activation of aryl group (d), de-directing group and C-8' configuration inversion (e), esterification of side chain (f) and other methods to obtain broom-like Synthetic derivatives G3-G5 of Camellia lactones, the specific six-step chemical reaction is:
[0078]
[0079] (a) Preparation of intermediate B1 by intermolecular 2+2 photochemical reaction: Add precursor compound A (200mg per tube) to two 15mL quartz tubes, then add 10mL of analytically pure acetonitrile to dissolve the sample, and then add 1mL of methyl acrylate Esters were reacted in a photoreactor with a wavelength of about 300nm for 1 day. The sample was evaporated to dryness...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com