N-containing bis-heterocyclic amide compounds and their application as immunosuppressants
A technology of heterocyclic amides and compounds, which is applied in the field of medical immunology, can solve problems such as poor targeting, and achieve the effect of simple preparation method, simple structure and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
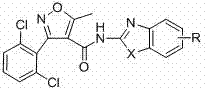
[0035] Example 1: Preparation of 3-(2,6-dichlorophenyl)-5-methyl-N-2-(6-methanesulfonylbenzothiazolyl)isoxazole-4-carboxamide:
[0036] Under anhydrous and oxygen-free conditions (argon protection), 0.347 g (1.2 mmol) of 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride (product 2) was dissolved in in 4 mL of dry dichloromethane and placed in a pre-dried constant pressure funnel. A dry 50mL three-neck round bottom flask was added with 0.212g (1mmol) of 2-amino-6-methylsulfonylbenzothiazole, followed by 5mL of anhydrous dichloromethane and 1mL of anhydrous N,N-dimethylmethane Amide (DMF) was dissolved as a reaction solubilizer, and 0.25 mL of anhydrous triethylamine was used as an acid binding agent. A solution of 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride in 4 mL of dichloromethane was added dropwise to a round bottom flask placed in a cryogenic reactor. The temperature was kept at 40-45°C, and after the dropwise addition was completed, the react...
Embodiment 2
[0037] Example 2: Preparation of 3-(2,6-dichlorophenyl)-5-methyl-N-[2-(6-ethylbenzothiazole)isoxazole]-4-carboxamide:
[0038] Under anhydrous and oxygen-free conditions (argon protection), 0.178g (1mmol) of 2-amino-6-ethylbenzothiazole was placed in a three-neck round bottom flask, and 5mL of anhydrous dichloroethane and 1mL of Anhydrous DMF was used as the reaction solvent, and 0.25 ml of anhydrous triethylamine was used as the acid binding agent. The mixture was mixed at a low temperature and cooled to 0° C. and kept for 15 minutes. A solution of 0.347 g (1.2 mmol) of 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride in dichloromethane was added dropwise to the reaction system. After stirring at low temperature for 30 minutes, it was transferred to an oil bath at 60° C. to react for 18 hours. The solution changed from yellow to tan. The progress of the reaction was monitored by thin layer chromatography (developing solvent petroleum ether:ethyl acetate=1:5). A...
Embodiment 3
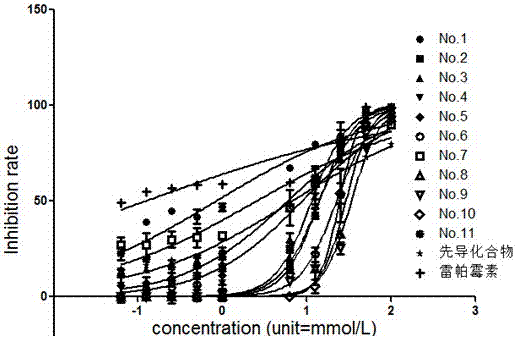
[0041] Example 3: CCK-8 method compound 4a, i.e. 3-(2,6-dichlorophenyl)-5-methyl-N-2-(6-methanesulfonylbenzothiazolyl)isoxazole-4 - Inhibition experiment of formamide on Jurkat cells.
[0042] For Jurkat cells in log phase condition, the supernatant was discarded after centrifugation, and 1 ml of fresh 10% FBS RPMI-1640 complete culture medium was added to gently pipet the cells to avoid air bubbles to prepare a single cell suspension. Count the cells, dilute the cell suspension in proportion to make the cell density reach 6×105 cells / ml, and mix well. Plating: First, add 100 μL of PBS to 36 wells around the experimental wells of a 96-well cell culture plate. Starting from the B2 hole of the culture plate, 50 μL of cell suspension was added to the experimental wells in turn, and then 200 mmol / L, 100 mmol / L, 50 mmol / L, 25 mmol / L, 12.5 mmol / L and 2 mmol were added to each experimental well. / L, 1mmol / L, 500mmol / L, 250nmol / L, 125nmol / L of the drug solution containing N-heterocy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com