Furan ring derivative and application thereof in preparation of medicine with immunosuppressive activity
A technology of immunosuppression, derivatives, applied in the fields of medicine and microbiochemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
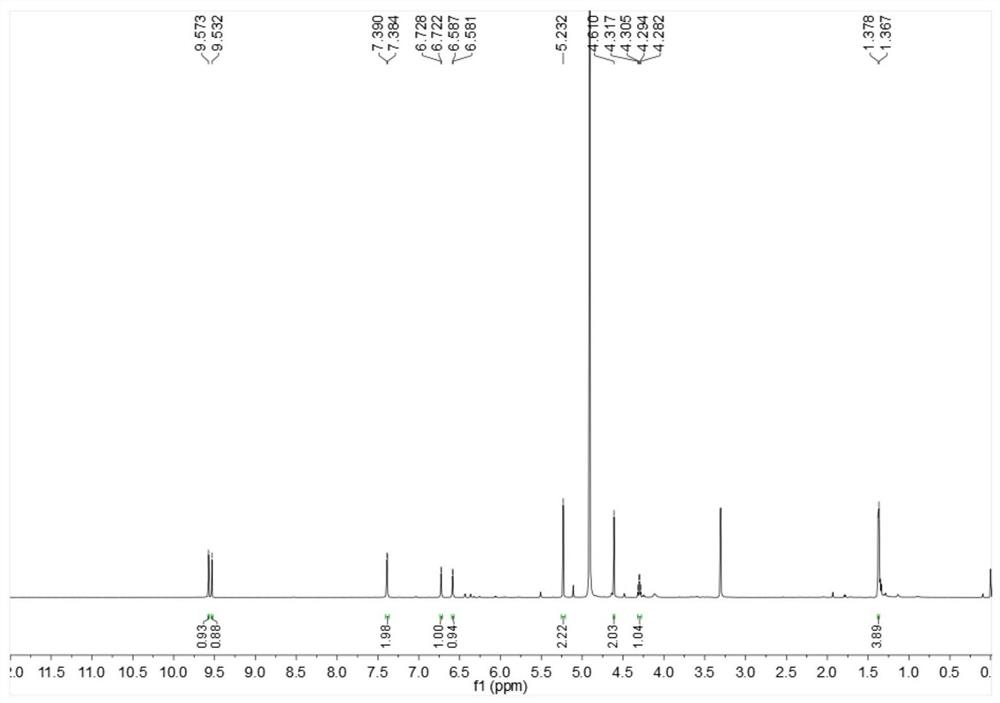
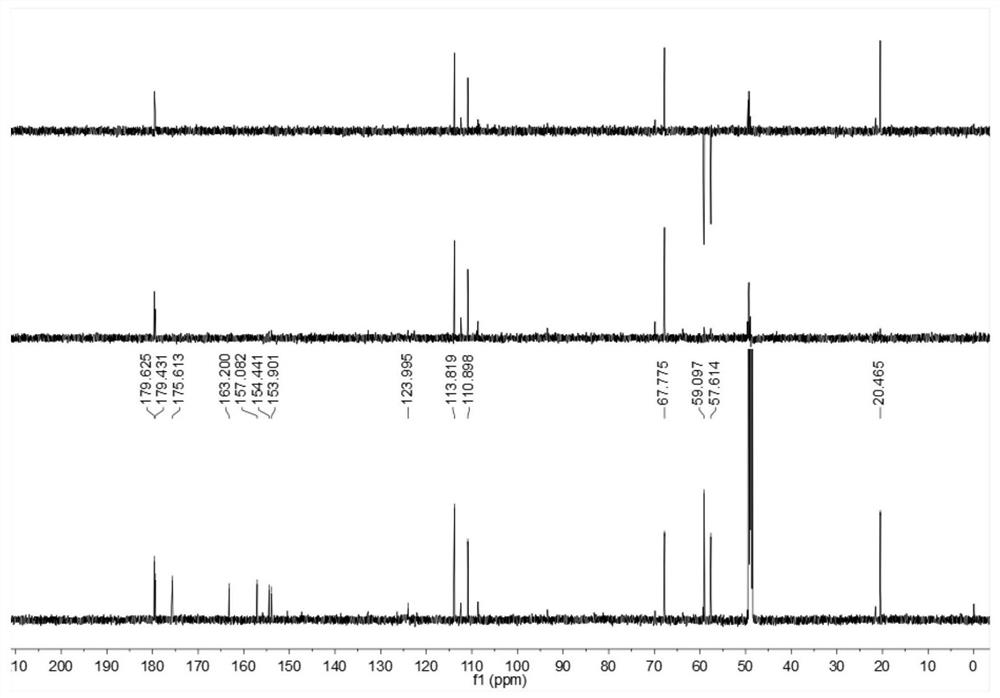
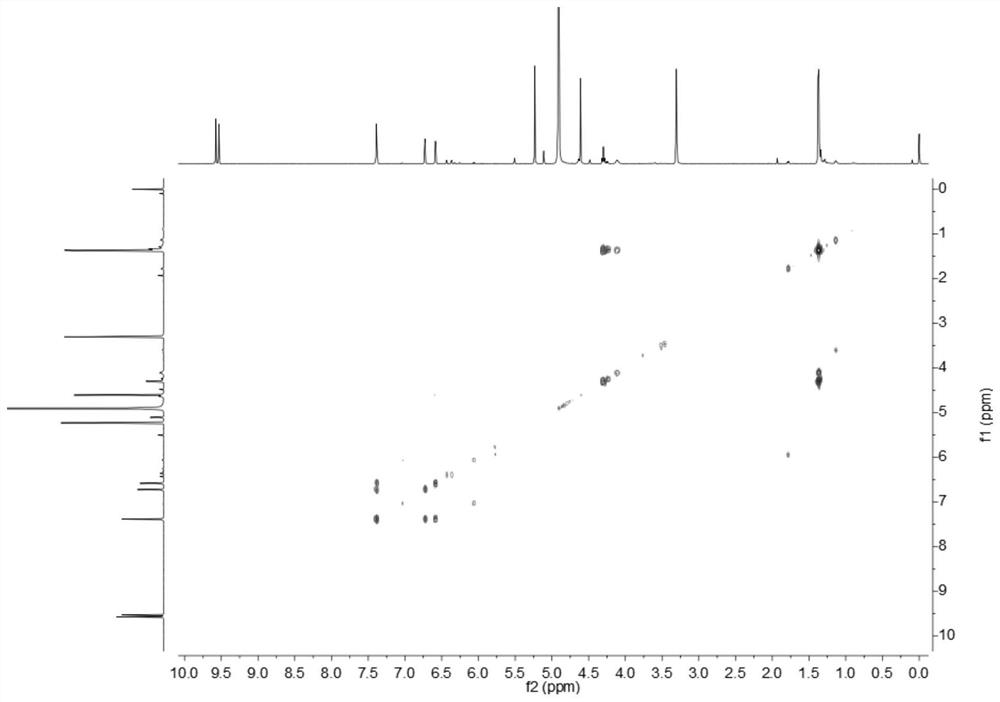
[0026] Embodiment 1: The compound described in the claims and the description of the invention - a furan ring derivative is obtained as follows:
[0027] Compound separation and purification process:
[0028] Glucose-peptone liquid culture medium is 80L in total, among which glucose (50g), peptone (10g), potato (200g), KH 2 PO 4 (10g), yeast powder (10g), evenly distributed in the conical flask (250mL × 640 bottles, 125mL per bottle), inoculate 5ml of culture medium in each bottle with a concentration of 6 × 10 7 cfu / ml of MHHNU7966-IS bacterial suspension, then cultured on a shaker, cultured and fermented in the dark at a constant temperature of 24°C and rotated at 150rpm for 30 days, and then used a centrifuge to filter 80L of Cordyceps xuefeng liquid into fermentation broth and solid Mycelia, and then utilize rotary evaporator and vacuum pump and low-temperature cooling circulating pump (the same below, decompression concentration device) to distill the filtrate (i.e. "fe...
Embodiment 2
[0037] Example 2: Determination of the immunosuppressive activity of the compound prepared in Example 1 using the non-specific toxicity and proliferation response experiments of mouse lymphocytes
[0038] (1) Preparation of mouse spleen lymphocytes:
[0039] The mice were sacrificed by devertebralization, and their spleens were aseptically removed to prepare a single cell suspension, and the erythrocytes were removed with erythrocyte lysate to adjust the cell concentration.
[0040] (2) Compound concentration processing:
[0041] All test compounds were dissolved in DMSO (dimethyl sulfoxide), and the stock solution concentration was 40 mM. For the detection of cytotoxicity, 200 μM of the test compound was the highest concentration, and 2.5-fold serial dilution was set up with 10 concentrations: 200, 80, 32, 12.8, 5.12, 2.048, 0.82, 0.33, 0.13, 0.05 μM; cyclosporine A ( Cyclosporin A, CsA) 20μM is the highest concentration, 2-fold serial dilution, set 10 concentrations: 20, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com