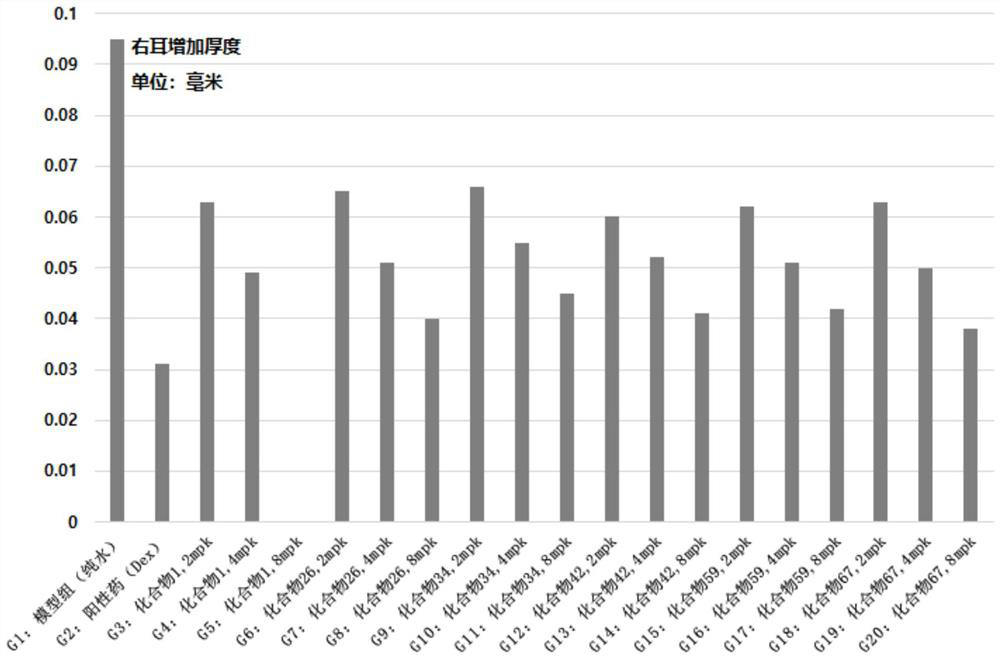
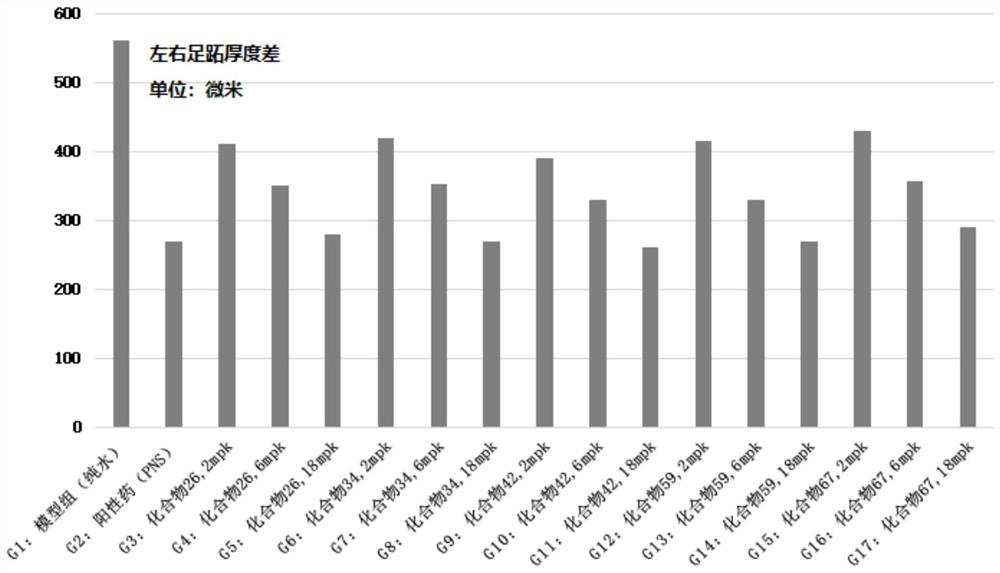
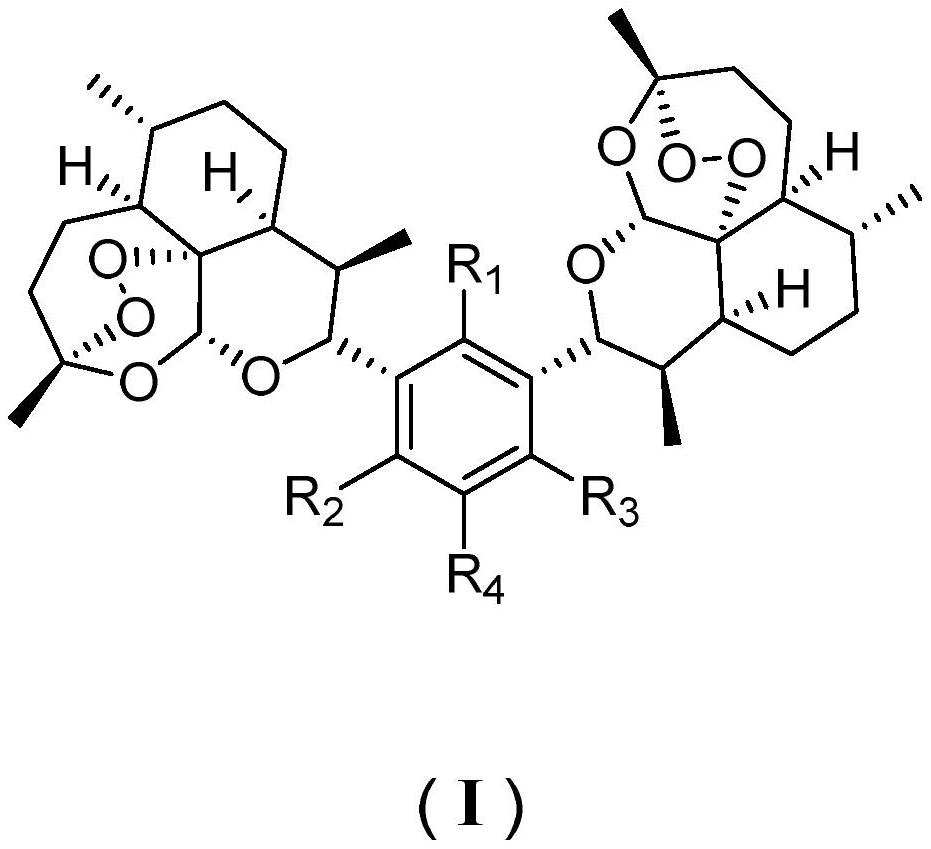
Preparation and application of bis-(10-deoxydihydroartemisinin)-phloroglucinol derivative
A technology of dihydroartemisinin and phloroglucinol, which is applied in the field of biomedicine, can solve the problems of high toxicity and narrow therapeutic window, and achieve the effects of low cytotoxicity, simple synthesis and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 compound 14
[0063]
[0064] Under nitrogen protection, dihydroartemisinin (1.30 g, 4.6 mmol) was dissolved in 20 mL of dry dichloromethane, the reaction system was cooled to -30 °C, and DAST (diethylaminosulfur trifluoride, 0.66mL, 4.6mmol), and then continued to stir at -30°C for 1 hour, and monitored the reaction by TLC until the starting material was consumed to obtain intermediate 14-1. Then directly add 1,3,5-trimethoxybenzene (1.55g, 9.2mmol) to the reaction solution and cool down to -78°C, then slowly add BF 3 ·Et 2 O (692 μL, 5.5 mmol), and then the reaction temperature was gradually increased from -78°C to room temperature. TLC monitoring until the end of the reaction, with saturated NaHCO 3 Aqueous solution (20 mL) was used to quench the reaction, the organic phase was separated and the aqueous phase was extracted twice with 10 mL of dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate,...
Embodiment 2
[0070] The preparation of embodiment 2 compound 15
[0071]
[0072] Under nitrogen protection, compound 1 (100 mg, 0.15 mmol) was dissolved in 15 mL of acetone, 3-bromopropene (66 μL, 0.75 mmol) and potassium carbonate solid (62 mg, 0.45 mmol) were added, and the reaction system was heated to 70 ° C, refluxed 6 hours. TLC monitors the reaction until the end of the reaction, the reaction solution is diluted with 30 mL of ethyl acetate, then washed twice with saturated brine, the organic phase is dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue is purified by silica gel column chromatography (elution Agent: petroleum ether / ethyl acetate=5 / 1), 78 mg of white solid product 15 was obtained, and the yield was 74.7%.
[0073] 1 H NMR (400MHz, DMSO-d 6 )δ8.54(d, J=3.4Hz, 2H), 6.02(q, J=10.9Hz, 2H), 5.66(s, 2H), 5.41(dd, J=17.3, 1.8Hz, 1H), 5.26( dd,J=10.7,1.7Hz,1H),5.15(d,J=11.2Hz,1H),5.08(d,J=11.2Hz,1H),4.47(dd,J=12.3,4.6Hz,2H), 2.65-...
Embodiment 3
[0076] The preparation of embodiment 3 compound 16
[0077]
[0078] Compound 2 (190mg, 0.26mmol) was dissolved in a mixed solution of 20mL methanol, 20mL tetrahydrofuran, and 2mL water and cooled to 5°C with an ice-water bath, then LiOH solid (61mg, 2.6mmol) was added and stirred at 5°C for 1 hour . After the reaction was monitored by TLC, 0.05M dilute hydrochloric acid was added dropwise until the pH of the reaction solution was about 5, concentrated under reduced pressure at room temperature to remove most of the solvent, the residue was diluted with 20 mL of water, and extracted twice with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (eluent: dichloromethane / methanol=15 / 1) to obtain 113 mg of white solid product 16, yield 61.8% .
[0079] 1 H NMR (400MHz, DMSO-d 6 )δ8.54(d, J=2.4Hz, 2H), 5.85(s, 1H), 5.63(d, J=15.9Hz, 2H), 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com