Method for preparing 2,3-butanedione from paraformaldehyde
A technology of paraformaldehyde and diacetyl, applied in the preparation of heterocyclic compounds, organic chemistry, etc., can solve the problems of unavailable raw materials, high technical content requirements, difficult industrial application, etc., and achieve novel synthetic routes, simple process operation, The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
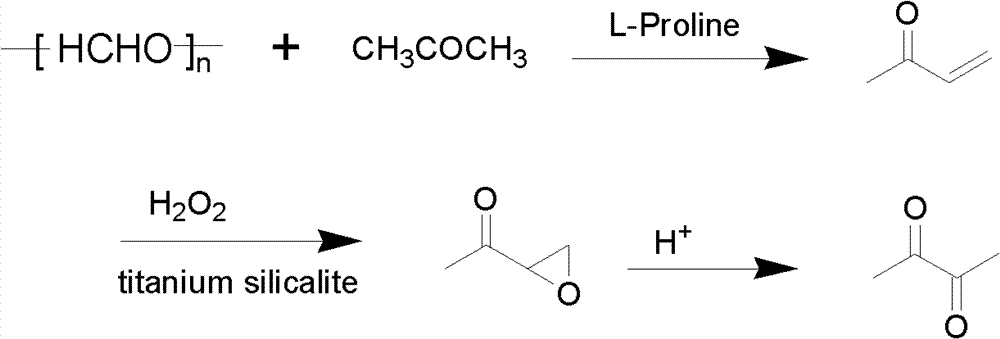
[0028] 20g acetone, 3.448g paraformaldehyde, 0.004molHCl, 1g L-proline, join together in the self-generating pressure reactor (the pressure that produces as temperature rises), start stirring and slowly heat up, and the reaction temperature is controlled at 100 DEG C, reaction time 2 hours, naturally cooled after the reaction, analyzed by gas chromatography, the conversion rate of paraformaldehyde was 99%, and the selectivity of methyl vinyl ketone was 96.7%.
[0029] Using synthesized methyl vinyl ketone as raw material, adjust the pH of the solution to 8, add 0.1 g of titanium-silicon molecular sieve catalyst, slowly add 16.39 g of hydrogen peroxide dropwise in a water bath at 55°C, and react with sodium bisulfite after 3 hours Adjust the pH of the solution to 1, continue to increase the reaction temperature to 80° C., and react for 2 hours. Analyzed by gas chromatography, the conversion rate of methyl vinyl ketone is 94.7%, and the selectivity of diacetyl is 85%.
Embodiment 2
[0031] 20g of acetone, 3.448g of paraformaldehyde, 0.004molHCl, and 2g of L-proline were added together into the self-generating pressure reactor, the stirring was started and the temperature was raised slowly, the reaction temperature was controlled at 100°C, and the reaction time was 2 hours. Cooling, analysis by gas chromatography, the conversion rate of paraformaldehyde is 99%, and the selectivity is 97%.
[0032] Using synthesized methyl vinyl ketone as raw material, adjust the pH to 8, add 0.1 g of titanium-silicon molecular sieve catalyst, slowly add 16.39 g of hydrogen peroxide dropwise in a water bath at 55°C, and adjust with sodium bisulfite after reacting for 3 hours pH = 1 (sodium bisulfite is added as a reducing agent to adjust the pH value of the solution) excess hydrogen peroxide, and the temperature is raised to 80° C. for 2 hours to prepare diacetyl. The conversion rate of methyl vinyl ketone was 97%, and the selectivity of diacetyl was 83%.
Embodiment 3
[0034] Add 20g of acetone, 1.724g of paraformaldehyde, 0.002molHCl, and 0.5g of L-proline together into the self-generating pressure reactor, start stirring and slowly raise the temperature, the reaction temperature is controlled at 100°C, and the reaction time is 2 hours. Cool naturally, analyze with gas chromatography, the conversion rate of paraformaldehyde is 99%, selectivity 87%.
[0035] Using synthetic methyl vinyl ketone as raw material, adjust pH=8, add 0.1g of titanium-silicon molecular sieve catalyst, slowly add 7.35g of hydrogen peroxide dropwise in a water bath at 55°C, and adjust with sodium bisulfite after reacting for 3 hours pH = 1 and excess hydrogen peroxide was reduced, and the temperature was raised to 80° C. for 2 hours to prepare diacetyl. The conversion rate of methyl vinyl ketone was 93.5%, and the selectivity of diacetyl was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com