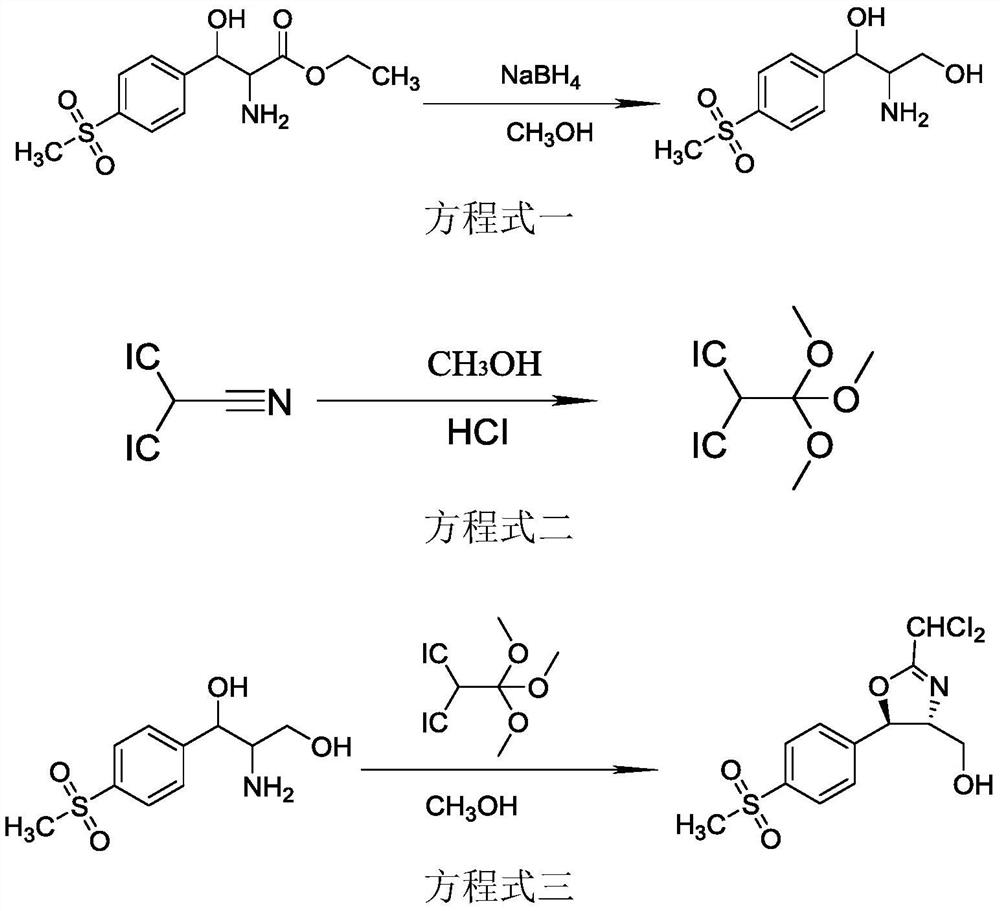
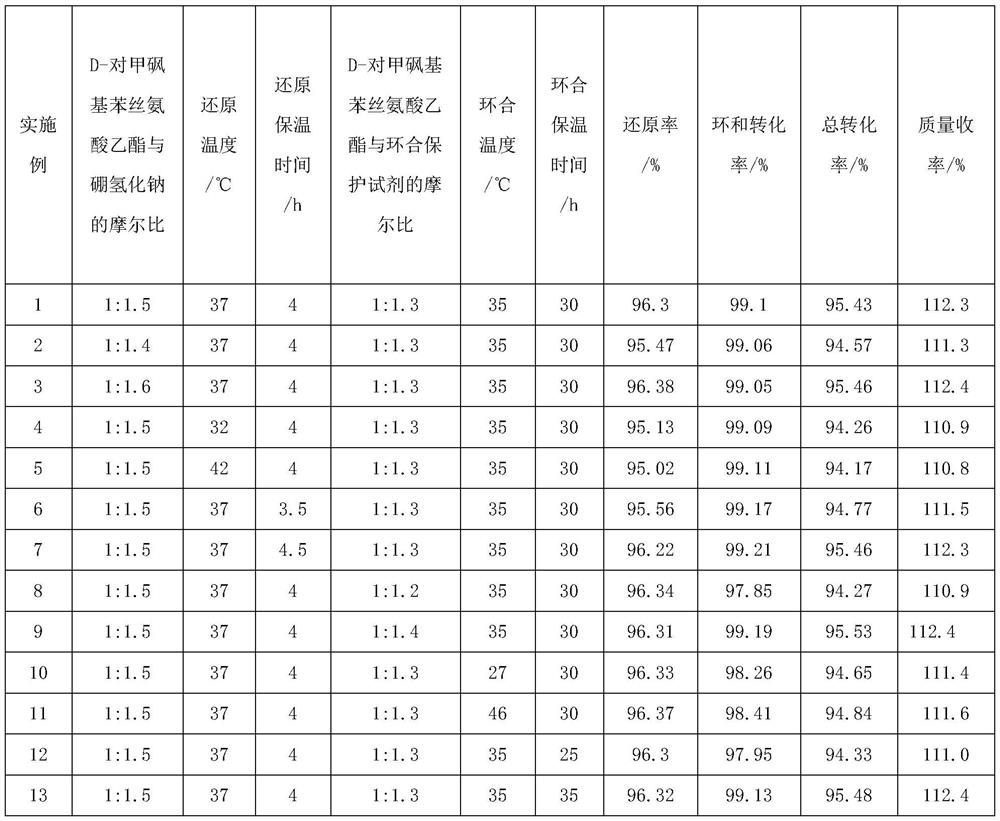
Florfenicol intermediate preparation method
A technology of florfenicol and intermediates, which is applied in the field of preparation of florfenicol intermediates, can solve problems such as poor control of process parameters, unsuitability for industrialized production, and low industrialization value, etc., so as to improve the total conversion rate , Novel synthetic route and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 500ml four-neck flask, add 300g of methanol and raise the temperature to 30°C, add 45.01g of D-p-thymphenylphenylserine ethyl ester, control the temperature at 30°C, add 8.89g of sodium borohydride, and feed the process of sodium borohydride , use ice water to cool down and control the reaction temperature at 37°C, then keep it warm in a water bath at 37°C, stir and react for 4 hours, after the heat preservation is over, use ice water to cool down to 5°C, add hydrochloric acid to adjust the pH value to 7.0, and weigh the total mass of the raw material solution , the sample was sent to the liquid phase to detect the external standard, and the reduction conversion rate was 96.30%. The reduction reaction obtained a mixed solution of the product D-threo-2-amino-1-p-thiamphenylphenyl-1,3-propanediol and boron ester. Set the temperature of the water bath to 75°C to carry out the distillation operation to recover the boron ester therein, recover until the solution bubbles ...
Embodiment 2
[0031]In a 500ml four-neck flask, add 300g of methanol and raise the temperature to 30°C, add 45.01g of D-p-thymphenylphenylserine ethyl ester, control the temperature at 30°C, add 8.29g of sodium borohydride, and feed the process of sodium borohydride , use ice water to cool down and control the reaction temperature at 37°C, then keep it warm in a water bath at 37°C, stir and react for 4 hours, after the heat preservation is over, use ice water to cool down to 5°C, add hydrochloric acid to adjust the pH value to 7.0, and weigh the total mass of the raw material solution , the sample was sent to the liquid phase to detect the external standard, and the reduction conversion rate was 95.47%. The reduction reaction obtained the product D-threo-2-amino-1-p-thiamphenylphenyl-1,3-propanediol and a mixed solution of boroester. Set the temperature of the water bath to 75°C to carry out the distillation operation to recover the boron ester therein, recover until the solution bubbles and...
Embodiment 3
[0033] In a 500ml four-necked flask, add 300g of methanol and raise the temperature to 30°C, add 45.02g of D-p-thymphenylphenylserine ethyl ester, control the temperature at 30°C, add 9.48g of sodium borohydride, and feed the process of sodium borohydride , use ice water to cool down and control the reaction temperature at 37°C, then keep it warm in a water bath at 37°C, stir and react for 4 hours, after the heat preservation is over, use ice water to cool down to 5°C, add hydrochloric acid to adjust the pH value to 7.0, and weigh the total mass of the raw material solution , the sample was sent to the liquid phase to detect the external standard, and the reduction conversion rate was 96.38%. The reduction reaction obtained a mixed solution of the product D-threo-2-amino-1-p-thiamphenylphenyl-1,3-propanediol and boron ester. Set the temperature of the water bath to 75°C to carry out the distillation operation to recover the boron ester therein, recover until the solution bubble...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com