2-chloro-3,3,3-trifluoropropylene preparation method
A kind of technology of trifluoropropene and cyclopropane, applied in the field of preparing 2-chloro-3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of gas phase fluorination catalyst
[0040] 1g of ZnCl 2 , 13g of Al(NO 3 ) 3 9H 2 O, 360g of 10% CrCl 3 The solution is obtained by co-precipitation method to obtain hydroxide, which is fired, and then 15ml of the mixed metal fluoride salt obtained by HF treatment is put into a Monel alloy reactor, and chlorine gas is introduced at 400°C for 4 hours. Residual chlorine was removed with nitrogen. Then, when the reaction temperature is lowered to a suitable temperature, the reaction gas can be introduced to carry out the reaction.
Embodiment 2
[0041] Embodiment 2: the gas phase preparation of HCFC-1233xf
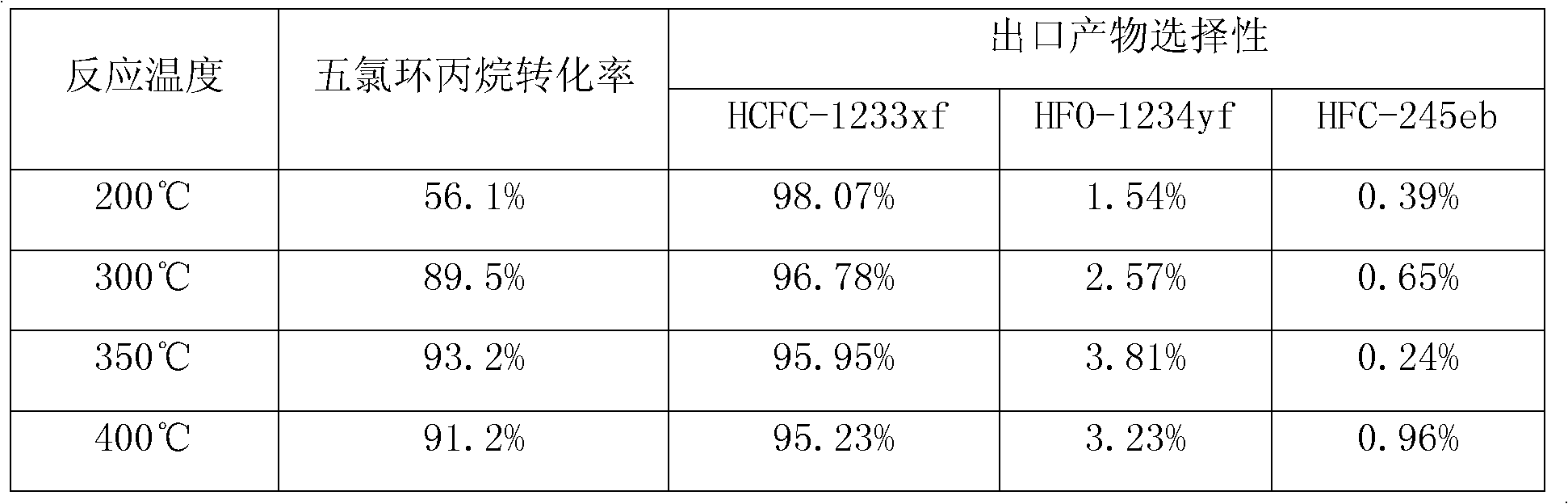
[0042] exist In the Monel alloy tube of the above-mentioned catalyzer, catalyzer is the catalyzer shown in embodiment 1, after processing as shown in embodiment 1, loading capacity is 50ml, earlier pentachlorocyclopropane and anhydrous HF are passed into preheating tank, After fully preheating and mixing at 200 ° C, it enters the reactor for reaction. The molar ratio of pentachlorocyclopropane and anhydrous HF is 6:1, and the contact time is 8.3 seconds. The product leaving the reactor is analyzed by GC-MS. The results are summarized in Table 1:
[0043] Table 1
[0044]
Embodiment 3
[0045] Embodiment 3: the gas phase preparation of HCFC-1233xf
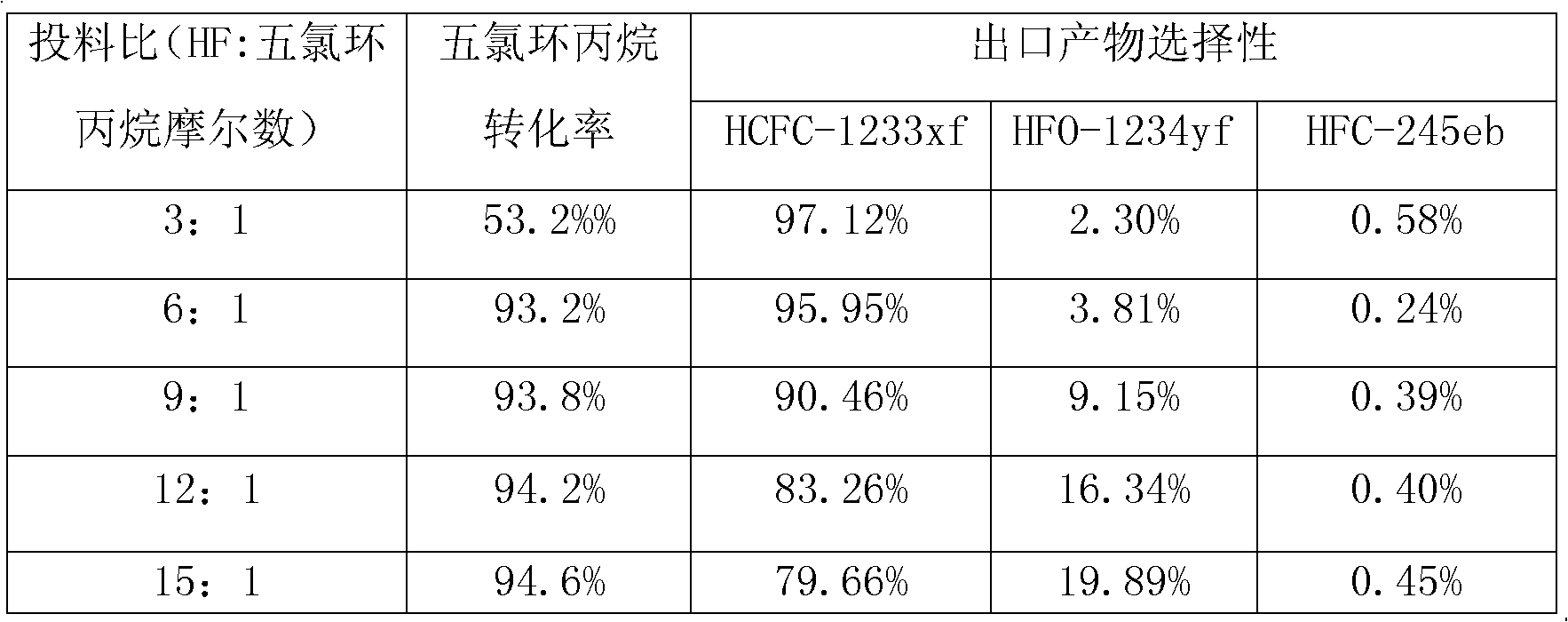
[0046] exist In the Monel alloy tube of the above-mentioned catalyzer, catalyzer is the catalyzer shown in embodiment 1, after processing as shown in embodiment 1, loading capacity is 50ml, earlier pentachlorocyclopropane and anhydrous HF are passed into preheating tank, After fully preheating and mixing at 200°C, add to the reactor for reaction, the reaction temperature is 350°C, the contact time is 8.3 seconds, the molar ratio of HF and pentachlorocyclopropane is changed, and the product leaving the reactor is analyzed by GC-MS. The results are summarized in Table 2:
[0047] Table 2
[0048]
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com