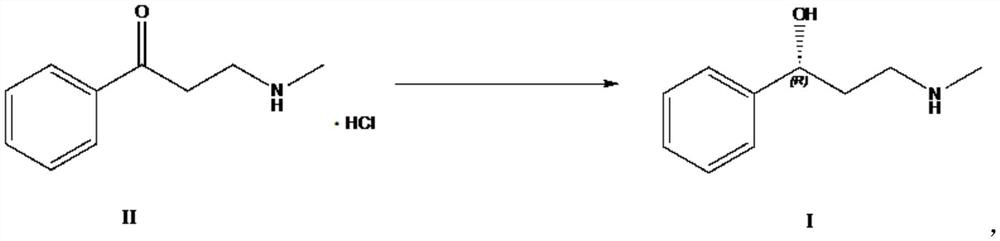
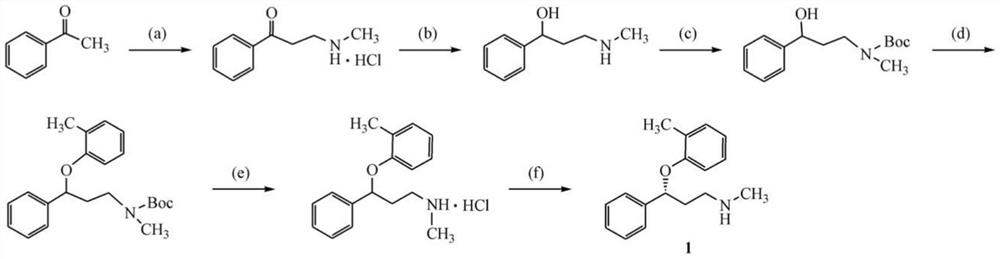
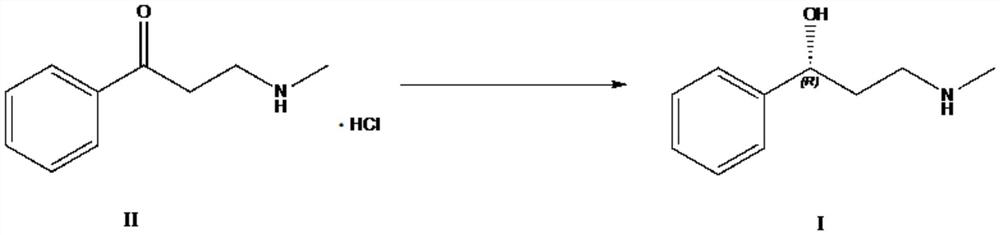
Biosynthesis method of atomoxetine intermediate and carbonyl reductase
A carbonyl reductase and biosynthesis technology, applied in the field of enzyme engineering, can solve the problems of low substrate solubility and reduced catalytic rate, and achieve the effects of improving enzyme catalytic efficiency, increasing solubility, and controlling costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 compound III
[0034] Take 30g of compound IV and add 300ml of acetone, stir at room temperature to dissolve and clarify, add 106.6g of sodium iodide and heat up to reflux reaction, about 10h, the unreacted amount of compound IV in HPLC is less than 5%, filter with suction, and concentrate the filtrate under reduced pressure to obtain 49.5g of compound III, add 150ml of n-hexane, recrystallize to get 33.34g, yield: 72%.
Embodiment 2
[0035] The preparation of embodiment 2 compound II
[0036] Take 33g of compound III prepared above, add 132ml THF, 264ml 25% monomethylamine aqueous solution, stir at room temperature, control in TLC, compound III reacts completely in about 20h, add THF under reduced pressure, add 5.6g sodium hydroxide and stir for 30min, add 400ml of toluene and 100ml of water were stirred and separated, and the aqueous phase was back-extracted with 200ml of toluene. The organic phases were combined and evaporated to dryness under reduced pressure to obtain 21.75g of crude product 3-methylamino-1-phenyl-1-propanone.
[0037] Add 60ml of ethanol to dissolve, add 17g of 30% hydrochloric acid ethanol dropwise, a large amount of solids precipitate out, stir and crystallize at about 0°C for 5h, filter with suction, and dry to obtain 17.4g of white solid, that is, compound II (3-methylamino-1-benzene base-1-propanone hydrochloride).
Embodiment 3
[0038] Embodiment 3 carbonyl reductase preparation
[0039] Escherichia coli TM1908 was inoculated into 10ml LB liquid medium with an inoculum size of 1%, and the medium contained 1mg kanamycin, and cultured on a shaker at 220rpm at 37°C for 8h to obtain a seed solution.
[0040] Put the above seed liquid into the fermented enzyme production medium, the inoculum size is 1%, add kanamycin at a final concentration of 10mg / 100mL to the fermentation medium, and culture at 37°C and 220rpm shaker to OD 600 Add IPTG at a final concentration of 0.5mM at 0.6-0.8, and induce at 18°C for 24h. After the induction of the fermentation broth, centrifuge at 12000rpm for 3min at 4°C to collect the cells. Wash the bacteria with 0.2 M pH7.0 PB buffer, resuspend, sonicate and freeze-dry to obtain carbonyl reductase freeze-dried enzyme powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com