Preparation method for 3-O-alkyl ascorbic acid
A technology of an alkyl ascorbic acid and a synthesis method, which is applied in the field of preparation of 3-O-alkyl ascorbic acid, can solve the problems of complex process products, complicated post-processing, unsuitable for large-scale production and the like, and achieves environmentally friendly process, simple and feasible process , the synthesis route is simple and novel effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
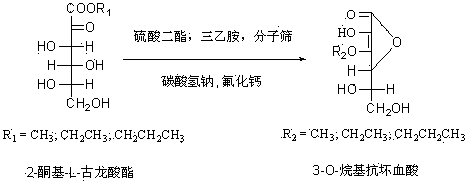
[0016] Under the protection of nitrogen, add 10.1 g (0.1 mol) of triethylamine to 50 ml of methanol, and slowly add 20.8 g (0.1 mol) of 2-keto-L-gulonate methyl ester to the above mixture under stirring. , Add 1 gram of molecular sieve, then add 12.6 grams (0.1 mol) of dimethyl sulfate dropwise, heat to 60 degrees and react for 3 hours, distill the methanol from the reaction solution under reduced pressure, and concentrate the viscous reaction solution in ethyl acetate After flushing, it quickly passes through a silica gel column (Flash column chromatography), and stops when the 2-keto-3-O-methyl-L-gulonate methyl ester is completely flushed out. Evaporate the solvent to obtain a yellow product. Under the protection of nitrogen, dissolve the obtained yellow product in 80 ml methanol, add 9.25 g (0.11 mol) sodium bicarbonate and 1.5 g (0.02 mol) calcium fluoride, reflux for 5 hours, and distill the methanol from the reaction solution under reduced pressure The concentrated visc...
Embodiment 2
[0018] Under the protection of nitrogen, add 10.1 g (0.1 mol) of triethylamine to 50 ml of ethanol, and slowly add 22.2 g (0.1 mol) of ethyl 2-keto-L-gulonate to the above mixture under stirring. , Add 1 gram of molecular sieve, and then add 15.4 grams (0.1 mol) of diethyl sulfate dropwise, heat to 78 degrees and react for 3 hours, distill the ethanol from the reaction solution under reduced pressure, and concentrate the viscous reaction solution in ethyl acetate After flushing, it quickly passes through a silica gel column (Flash column chromatography), and stops when the 2-keto-3-O-ethyl-L-gulonate ethyl ester is completely flushed out by the dot plate. Evaporate the solvent to obtain a yellow product. Under the protection of nitrogen, dissolve the obtained yellow product in 80 ml of ethanol, add 9.25 g (0.11 mol) of sodium bicarbonate and 1.5 g (0.02 mol) of calcium fluoride, reflux for 5 hours, and distill the ethanol from the reaction solution under reduced pressure The c...
Embodiment 3
[0020] Under nitrogen protection, add 10.1 g (0.1 mol) of triethylamine to 50 ml of ethanol, and slowly add 20.8 g (0.1 mol) of 2-keto-L-gulonate methyl ester to the above mixture under stirring. , Add 1 gram of molecular sieve, then add 22.2 grams (0.1 mol) of diethyl sulfate dropwise, heat to 78 degrees for 3 hours, distill the ethanol from the reaction solution under reduced pressure, and concentrate the viscous reaction solution in ethyl acetate After flushing, it quickly passes through a silica gel column (Flash column chromatography), and stops when the 2-keto-3-O-ethyl-L-gulonate methyl ester is completely flushed out. Evaporate the solvent to obtain a yellow product. Under the protection of nitrogen, dissolve the obtained yellow product in 80 ml of ethanol, add 9.25 g (0.11 mol) of sodium bicarbonate and 1.5 g (0.02 mol) of calcium fluoride, reflux for 5 hours, and distill the ethanol from the reaction solution under reduced pressure The concentrated viscous reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com