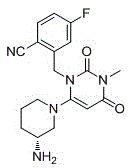
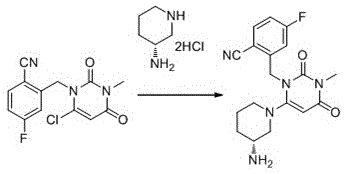
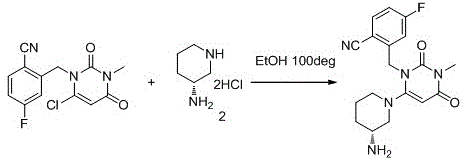
Preparation method of Trelagliptin
A single and mixed solvent technology, applied in organic chemistry and other fields, can solve the problems of increased impurities, many side reactions, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Add 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-methyl)-4-fluorobenzonitrile (2.930g, 0.010mol), dioxane (20ml), (R)-3-aminopiperidine bishydrochloride (2.070g, 0.012mol) and sodium bicarbonate (2.5g, 0.03mol) were added under stirring. The reaction was stirred at 75 degrees Celsius for 5 hours. LC-Ms detected that the reaction was complete and concentrated under reduced pressure. The crude product was refluxed with 20ml ethyl acetate for 1 hour, filtered while hot, and the filtrate was allowed to stand overnight. A solid precipitated out. The product was collected by filtration.
example 2
[0027] Add 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-methyl)-4-fluorobenzonitrile (29.3g, 0.1mol), dioxane (200ml), (R)-3-aminopiperidine bishydrochloride (20.7g, 0.12mol) and triethylamine (30.3g, 0.3mol) were added under stirring. The reaction was stirred at 70 degrees Celsius for 3 hours. LC-Ms detected that the reaction was complete. The reaction solution was cooled to room temperature, poured into 500 ml ice water, stirred at 5-10 degrees Celsius for 2 hours, filtered, and the solid was collected to obtain the product.
example 3
[0029] Add 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-methyl)-4-fluorobenzonitrile (2.930g, 0.010mol), acetonitrile (15ml), (R)-3-aminopiperidine bishydrochloride (2.07g, 0.012mol) and potassium carbonate (4.14g, 0.03mol) were added with stirring. The reaction was stirred at 75 degrees Celsius for 5 hours. LC-Ms detected that the reaction was complete. The reaction solution was cooled to room temperature, poured into 50 ml ice water, stirred at 5-10 degrees Celsius for 2 hours, filtered, and the solid was collected to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com