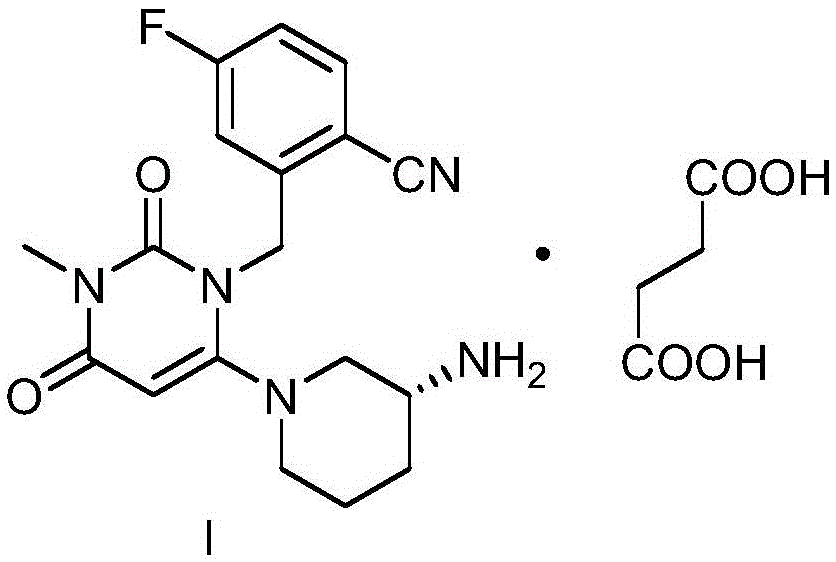
Synthetic method of trelagliptin, trelagliptin synthesized through method and trelagliptin synthesis intermediate
A synthetic method and intermediate technology, applied in the synthesis of trexagliptin, the field of synthetic intermediates of trexagliptin, can solve unfavorable safety production and environmental protection, unstable cost of benzyl bromide intermediates, and strong irritation of intermediates and other problems, to achieve the effect of reducing synthesis cost, low price and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
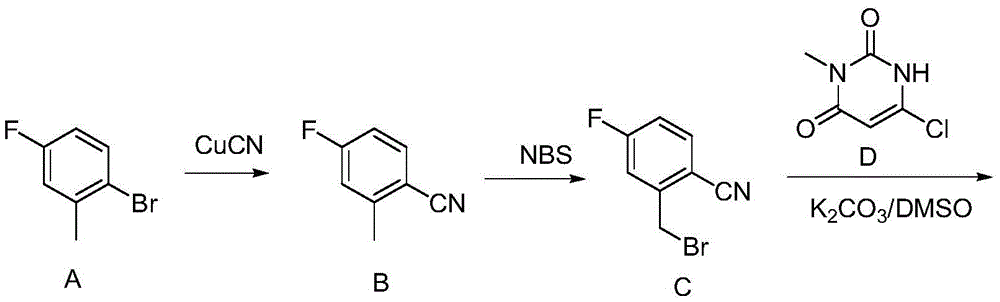
[0047] The synthetic method of Trexagliptin is as follows:
[0048] Step 1: Synthesis of Intermediate-1 (2-chloromethyl-4-fluorobenzonitrile)
[0049] Mix 21.2 g (0.14 mol) of 2-hydroxymethyl-4-fluorobenzonitrile and 80 g of thionyl chloride under stirring at room temperature, gradually raise the temperature to 70° C., stir and reflux for 2 hours, and TLC detects that the reaction is complete. Recover excess thionyl chloride, concentrate until there is no liquid drop to obtain intermediate 1 (2-chloromethyl-4-fluorobenzonitrile), add 2 times the mass volume of toluene, and set aside.
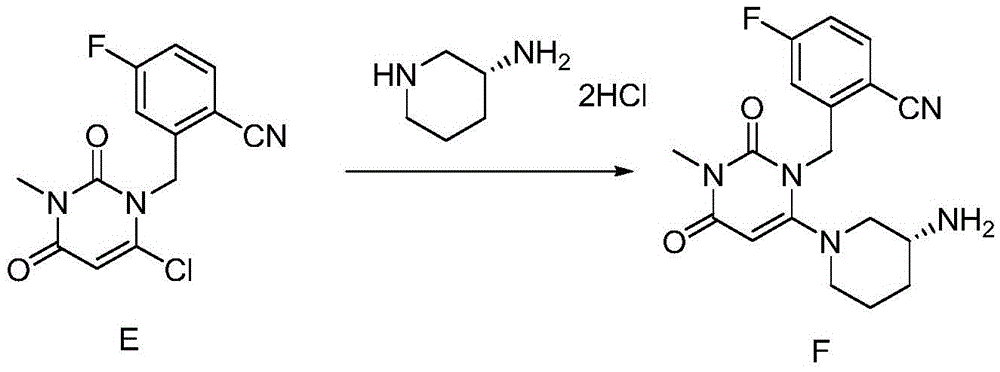
[0050] Step 2: Intermediate-2(2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4- Synthesis of fluorobenzonitrile).
[0051] Add 22.5g (0.14mol) of 6-chloro-3-methyluracil and 29mL of N,N-diisopropylethylamine (DIPEA) into 70mL of N-methylpyrrolidone, and stir at room temperature for 30min. Slowly add 23.8 g (0.14 mol) of toluene solution of Intermediate-1 (intermediate 1...
Embodiment 2
[0055] The synthetic method of Trexagliptin is as follows:
[0056] Step 1: Synthesis of Intermediate-1 (2-chloromethyl-4-fluorobenzonitrile)
[0057] At room temperature, 21.2 g (0.14 mol) of 2-hydroxymethyl-4-fluorobenzonitrile and 74.2 g of thionyl chloride were mixed under stirring, and the temperature was gradually raised to 75° C., stirred and refluxed for 5 hours, and the reaction was complete by TLC. Recover excess thionyl chloride, concentrate until there is no liquid drop to obtain intermediate 1 (2-chloromethyl-4-fluorobenzonitrile), add 2 times the mass volume of toluene, and set aside.
[0058] Step 2: Intermediate 2 (2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluoro Synthesis of benzonitrile).
[0059] Add 22.5g (0.14mol) of 6-chloro-3-methyluracil and 29mL of N,N-diisopropylethylamine (DIPEA) into 70mL of N-methylpyrrolidone, and stir at room temperature for 30min. Slowly add 23.8 g (0.14 mol) of toluene solution of Intermediate 1...
Embodiment 3
[0063] The synthetic method of Trexagliptin is as follows:
[0064] Step 1: Synthesis of intermediate 1 (2-chloromethyl-4-fluorobenzonitrile)
[0065] Mix 21.2 g (0.14 mol) of 2-hydroxymethyl-4-fluorobenzonitrile and 126 g of thionyl chloride under stirring at room temperature, gradually raise the temperature to 70° C., stir and reflux for 2 hours, and TLC detects that the reaction is complete. Recover excess thionyl chloride, concentrate until there is no liquid drop to obtain intermediate 1 (2-chloromethyl-4-fluorobenzonitrile), add 2 times the mass volume of toluene, and set aside.
[0066] Step 2: Intermediate 2 (2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-4-fluoro Synthesis of benzonitrile).
[0067] Add 22.5g (0.14mol) of 6-chloro-3-methyluracil and 29mL of N,N-diisopropylethylamine (DIPEA) into 70mL of N-methylpyrrolidone, and stir at room temperature for 30min. Slowly add 38.02 g (0.224 mol) of toluene solution of Intermediate 1 (Intermedia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com