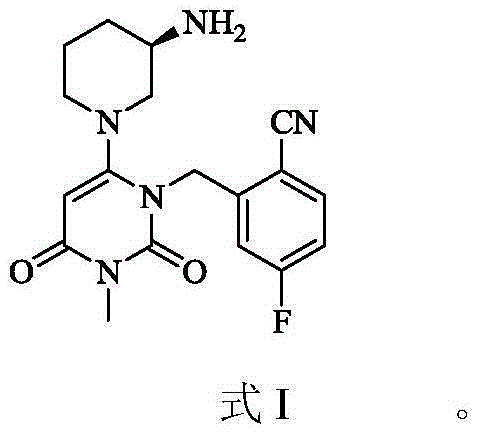
New crystal forms of trelagliptin, and preparation methods and application of crystal forms
A technology of crystal forms and uses, applied in the field of organic chemistry, can solve problems such as undisclosed solid forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] The preparation of embodiment 1 Trexagliptin crystal form F
[0101] Dissolve 7.0g of trexagliptin in 30ml of 1,2-propanediol at 95-100°C, stir and dissolve, cool to room temperature and let stand overnight, filter with suction, beat the filter cake with 20ml of ethyl acetate for about 0.5 hours Trexagliptin crystal form F was obtained.
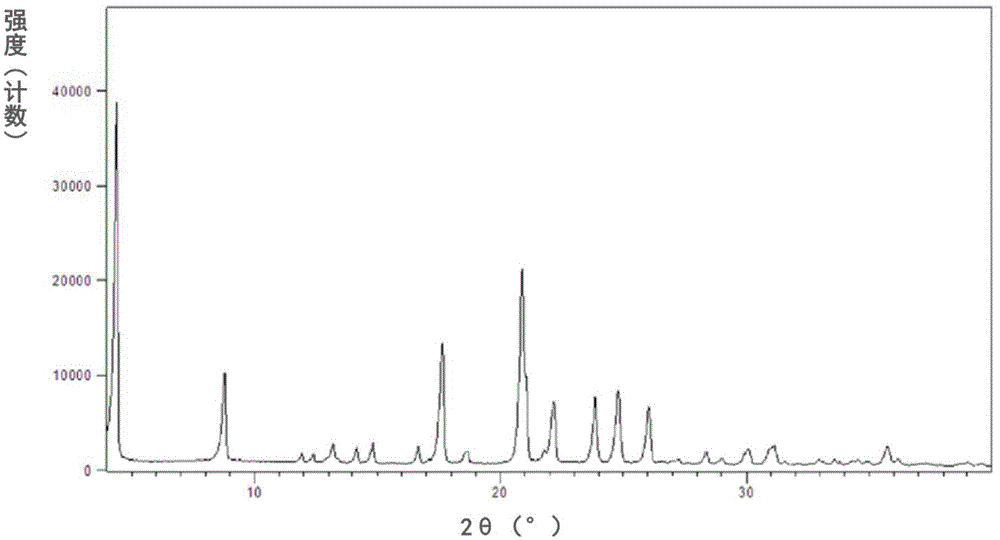
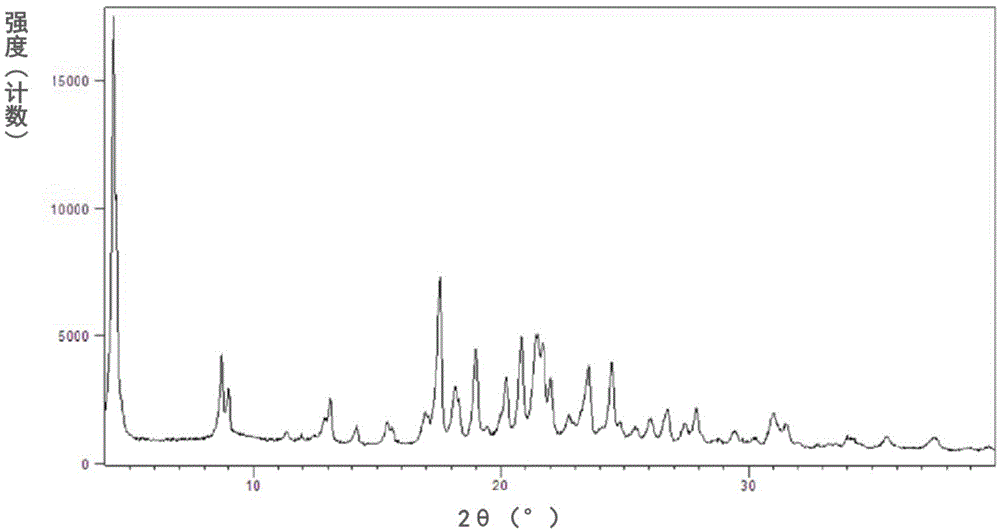
[0102] The measured powder X-ray diffraction pattern is shown in figure 1 , its measured value is as follows (get the measured value corresponding to the diffraction peak whose relative intensity is greater than 1%):
[0103]
[0104]
Embodiment 2
[0105] The preparation of embodiment 2 Trexagliptin crystal form F
[0106] At 80-90°C, dissolve 2.0g of trexagliptin in a mixed solvent of 2.5ml of 1,2-propanediol and 2.5ml of ethyl acetate, stir and dissolve, cool to room temperature naturally, and filter with suction to obtain trexagliptin crystals Type F.
Embodiment 3
[0107] The preparation of embodiment 3 Trexagliptin crystal form F
[0108] At 80-90°C, dissolve 6.0g of Trexagliptin in a mixed solvent of 4ml of 1,2-propanediol and 8ml of ethyl acetate, stir and dissolve, cool to room temperature naturally, filter with suction, and beat the filter cake with 30ml of ethyl acetate After about 1 hour, dry under reduced pressure at 30-40° C. to obtain trexagliptin crystal form F.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com