Salt, crystal and pharmaceutical composition of trelagliptin compound and applications thereof
A composition and drug technology, applied in the directions of drug combination, organic chemistry, organic chemistry methods, etc., can solve the problems such as the inability to maintain a crystalline state of troxagliptin succinate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Trexagliptin hemisuccinate hydrate crystal form I and its preparation
[0081] Take 15.00g (41.97mmol) trexagliptin and place it in a round-bottomed flask, add 200mL tetrahydrofuran and 100mL isopropanol, stir and heat to 40°C, the solid gradually dissolves during heating; weigh 2.47g (20.91mmol) of succinic acid Added to the trexagliptin solution, white solids precipitated after the addition, continued to stir for 60 minutes, stopped heating, naturally cooled to room temperature and stirred for 2 hours. After filtering, the filter cake was washed twice with isopropanol, sucked dry, and dried in vacuum at 40° C. to obtain trexagliptin hemisuccinate hydrate crystal form I.
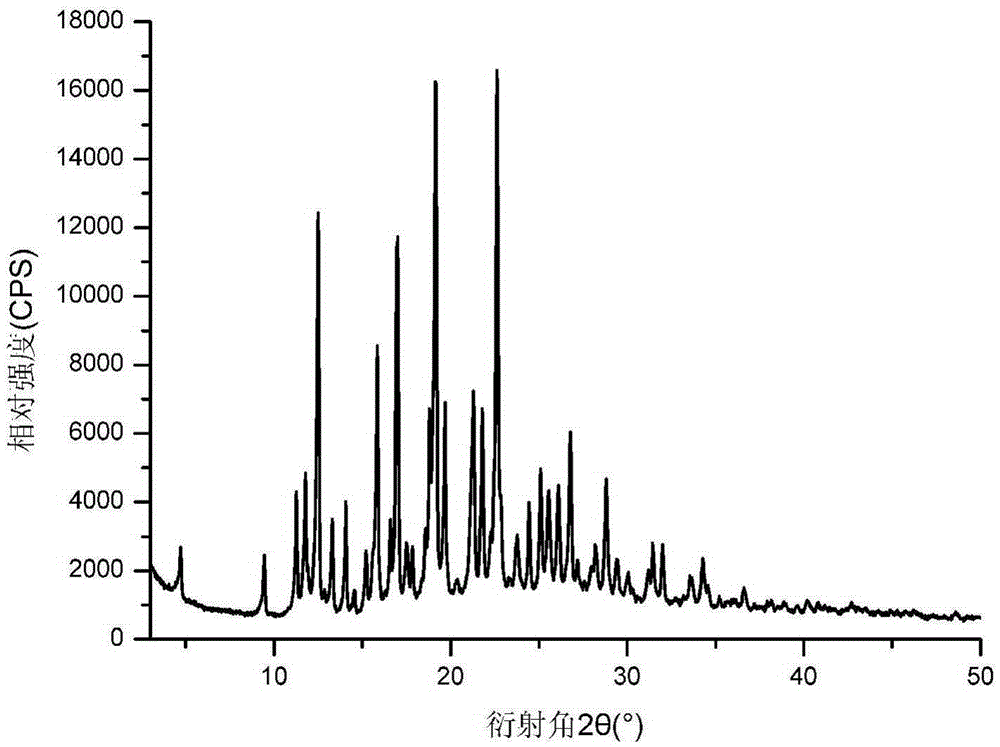
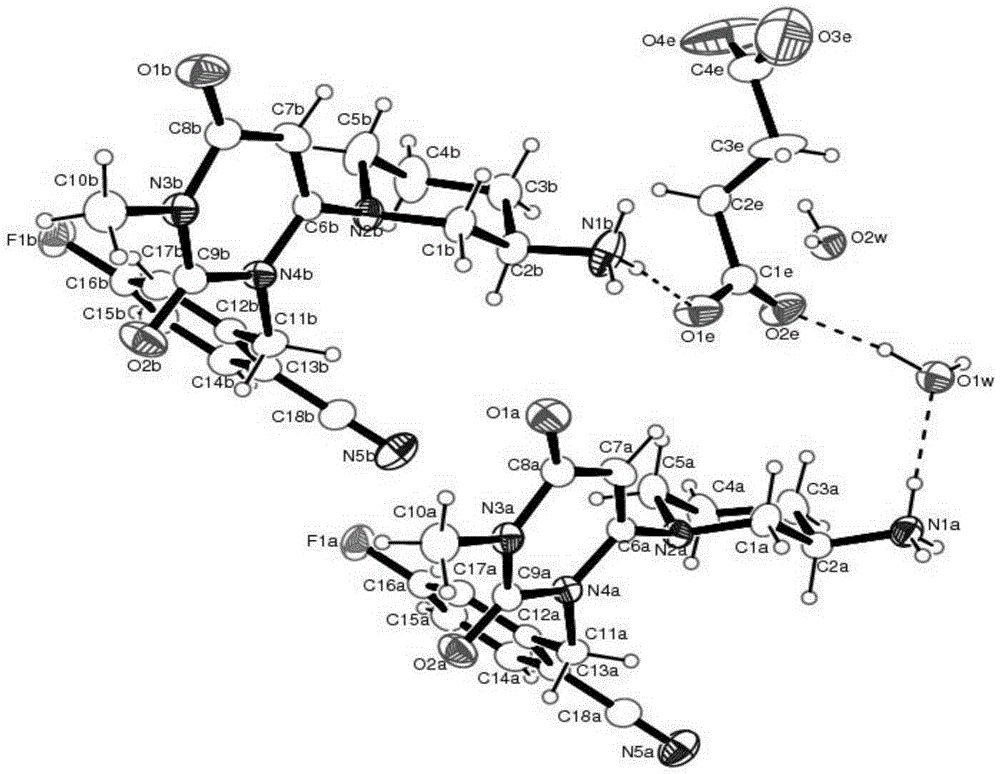
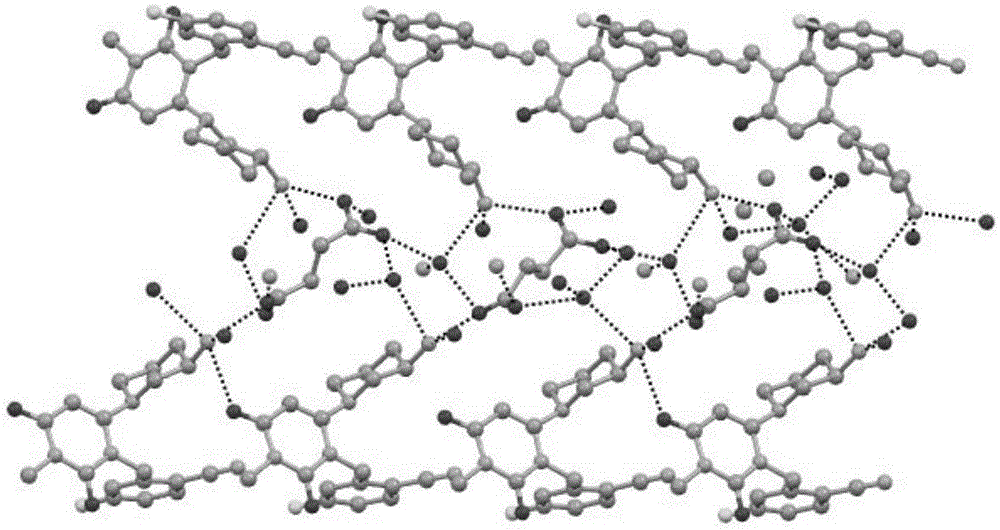
[0082] The crystalline form I of trexagliptin hemisuccinate hydrate prepared as above was identified via the triclinic unit cells of space group P1, which were characterized by single crystal X-ray structures at 296K The following parameters were analyzed analytically: α=85.883(2)°, β=8...
Embodiment 2
[0092] Example 2: Preparation of trexagliptin hemisuccinate hydrate crystal form I
[0093] Take 15.0g (41.97mmol) trexagliptin in a round bottom flask, add 150mL tetrahydrofuran, stir and heat to 50°C, the solid gradually dissolves during the heating process; weigh 2.47g (20.91mmol) of succinic acid and dissolve it in 50mL isopropyl alcohol; add the succinic acid solution to the trexagliptin solution, a white solid precipitates during the addition process, continue to stir for 20min, stop heating, naturally cool to room temperature and stir for 2h. After filtering, the filter cake was washed twice with isopropanol, sucked dry, and dried in vacuum at 50° C. to obtain trexagliptin hemisuccinate hydrate crystal form I.
[0094] After testing, the characteristic diffraction lines of X-ray powder diffraction are all the same as those in Example 1.
Embodiment 3
[0095] Example 3: Preparation of trexagliptin hemisuccinate hydrate crystal form I
[0096] Take 15.0g (41.97mmol) trexagliptin and place it in a round bottom flask, add 100mL tetrahydrofuran and 50mL isopropanol, stir and heat to 70°C, the solid gradually dissolves during the heating process; weigh 2.47g (20.91mmol) succinic acid Added to the trexagliptin solution, a white solid precipitated, continued to stir for 30 minutes, stopped heating, naturally cooled to room temperature and stirred for 2 hours. After filtering, the filter cake was washed twice with isopropanol, sucked dry, and dried in vacuum at 50° C. to obtain trexagliptin hemisuccinate hydrate crystal form I.
[0097] After testing, the characteristic diffraction lines of X-ray powder diffraction are all the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com