New solid forms of trelagliptin and manufacturing method and purpose thereof
A solid and crystalline technology, applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of undisclosed solid forms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
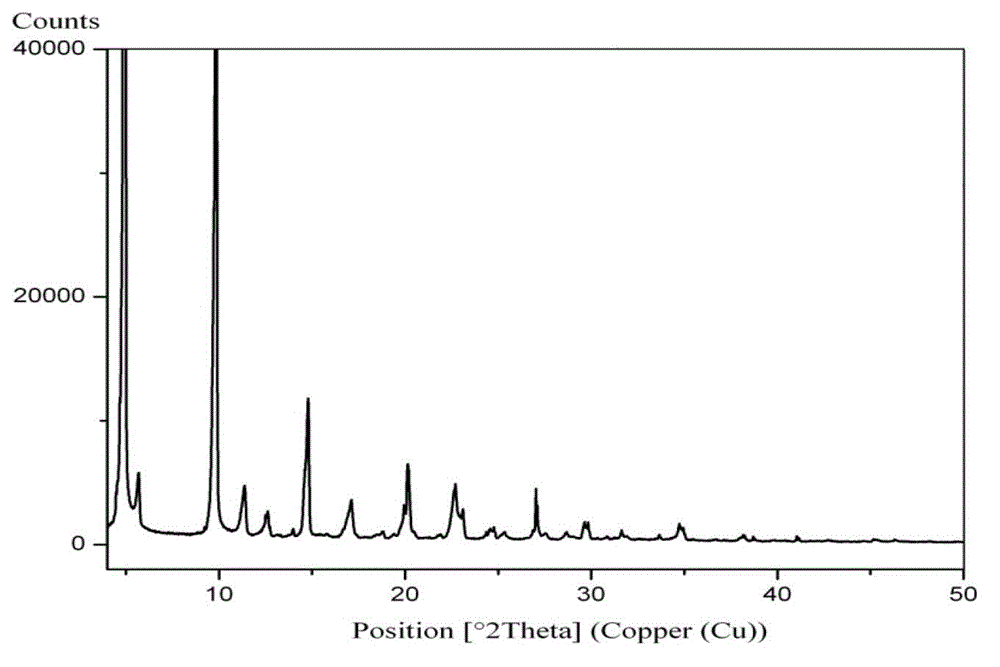
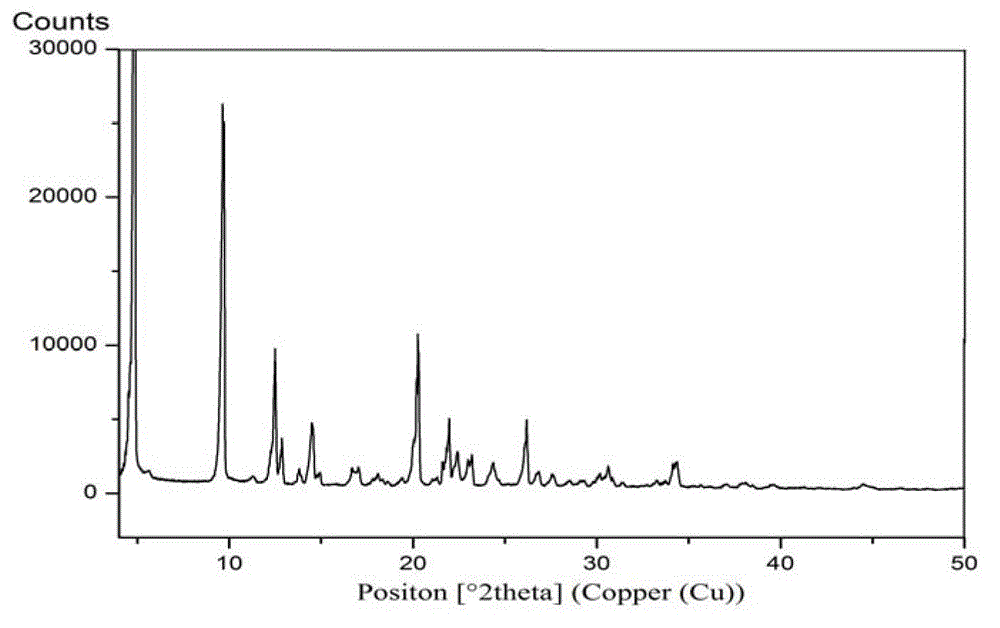
Image
Examples
Embodiment 1
[0137] The preparation of embodiment 1 Trexagliptin crystal form A
[0138] Dissolve 2.5g of Trexagliptin in 25ml of isopropanol at 75-78°C, cool to 5-10°C with stirring, filter with suction, and dry the resulting solid under reduced pressure at 45-50°C to obtain Quagliptin Gliptin crystal form A, white solid.
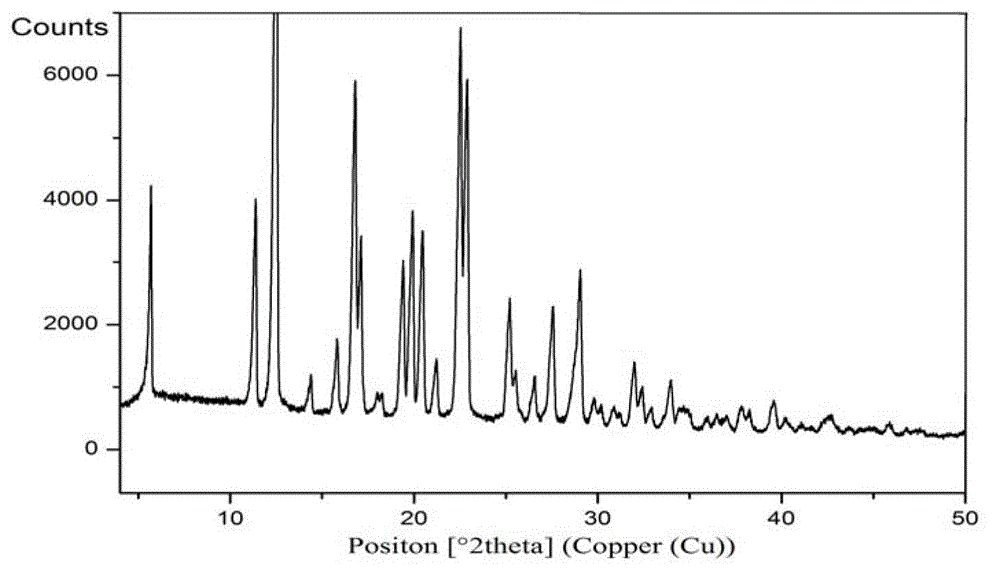
[0139] The measured powder X-ray diffraction pattern is shown in figure 1 , and its measured value is as follows (take the measured value corresponding to the diffraction peak whose relative intensity is greater than 1%):
[0140]
[0141]
Embodiment 2
[0142] The preparation of embodiment 2 Trexagliptin crystal form A
[0143] Dissolve 2.5 g of trexagliptin in 25 ml of tetrahydrofuran at 60-63°C, cool to 0-5°C while stirring, and filter with suction to obtain trexagliptin crystal form A as a white solid.
Embodiment 3
[0144] The preparation of embodiment 3 Trexagliptin crystal form A
[0145] Dissolve 2.5 g of Trexagliptin in 25 ml of ethyl acetate at 71-74°C, cool to 0-10°C while stirring, filter with suction, and dry the resulting solid under reduced pressure at 60-65°C to obtain Quagliptin Gliptin crystal form A, white solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com