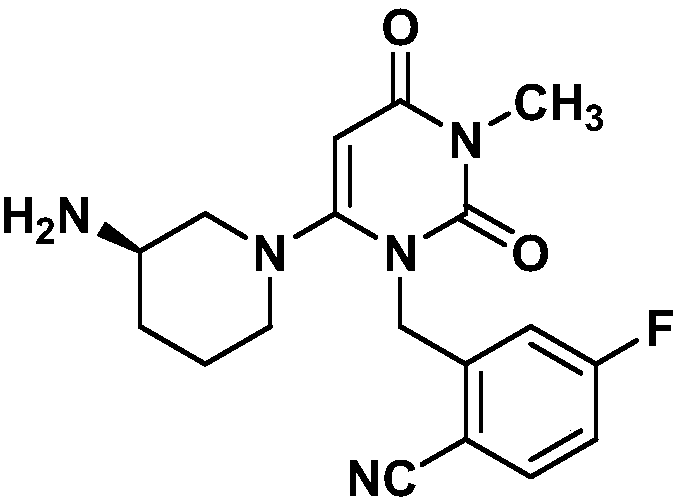
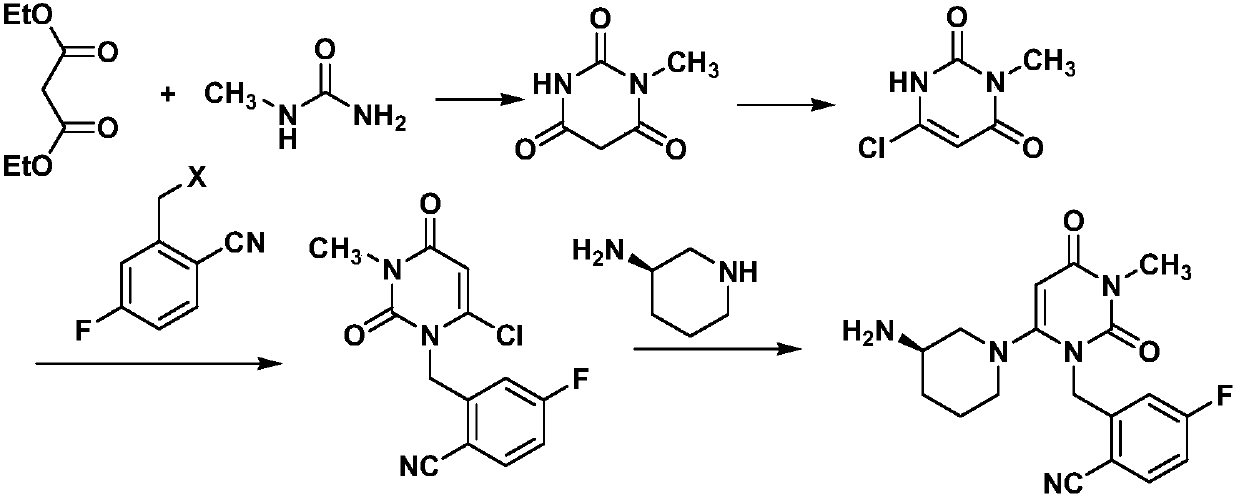
Preparation method for trelagliptin
A methyl-reaction technology, applied in the field of preparation of trelagliptin, can solve the problems of harsh reaction conditions and low yield, and achieve the effects of high product purity and yield, high yield and shortening reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] A preparation method of Trexagliptin, comprising the following steps:
[0029] (1) Preparation of 1-methylpyrimidine-2,4,6-trione
[0030] Methylurea (97.14 g, 1.31 mol), absolute ethanol 1.2 L, sodium metal (30.4 g, 1.32 mol) were added in batches, and stirred for 1 h. A solution of diethyl malonate (273.0 g, 1.70 mol) in 100 mL of absolute ethanol was slowly added dropwise to the reaction liquid, heated to reflux, and reacted for 24 h. The solvent was distilled off under reduced pressure, 1L of water was added, the pH was adjusted to 3-4 with 2N HCl, and the mixture was crystallized by cooling. Suction filtration, washing with water, and drying gave light yellow powder, 152.8 g of 1-methylpyrimidine-2,4,6-trione, yield 82%, mp.129-130°C.
[0031] (2) Preparation of 3-methyl-6-chlorouracil
[0032] 1-Methylpyrimidine-2,4,6-trione (80g, 0.564mol), 400g of phosphorus oxychloride, cooled to 0°C, controlled temperature at 0-5°C and stirred for 0.5h, heated to 60°C for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com