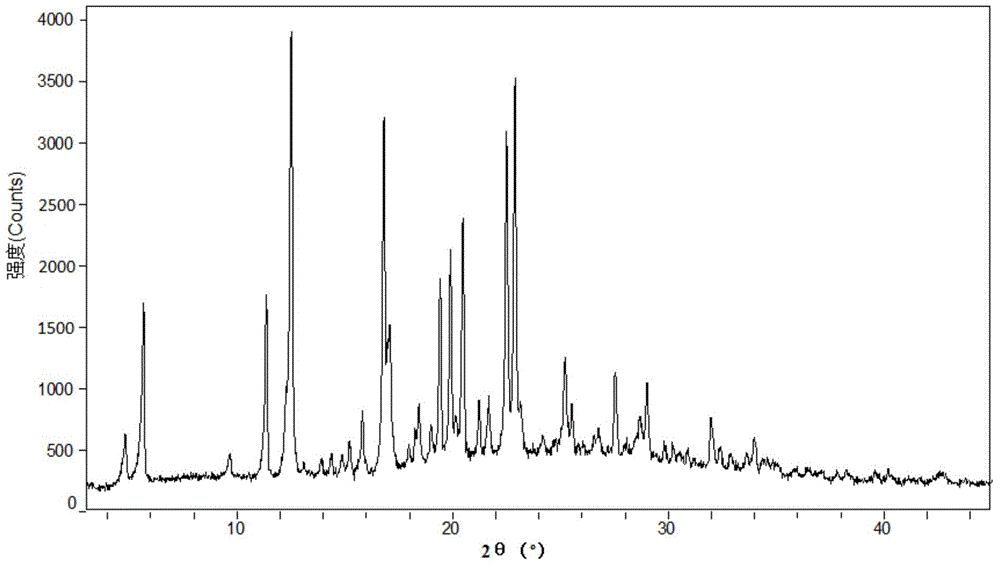
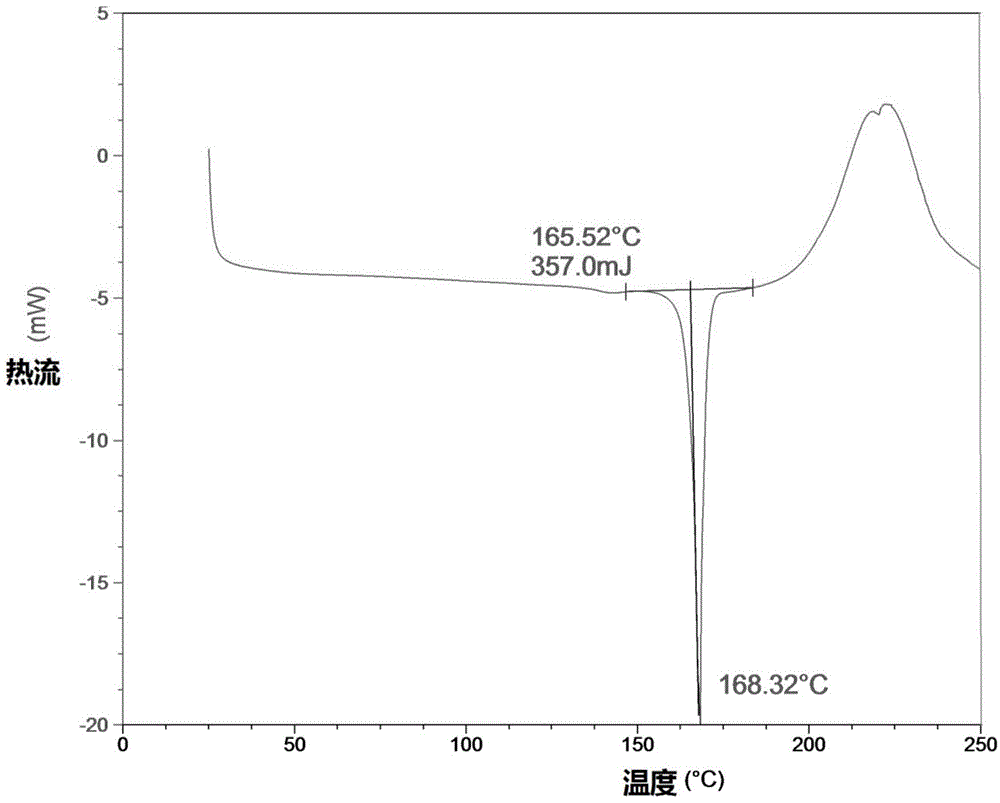
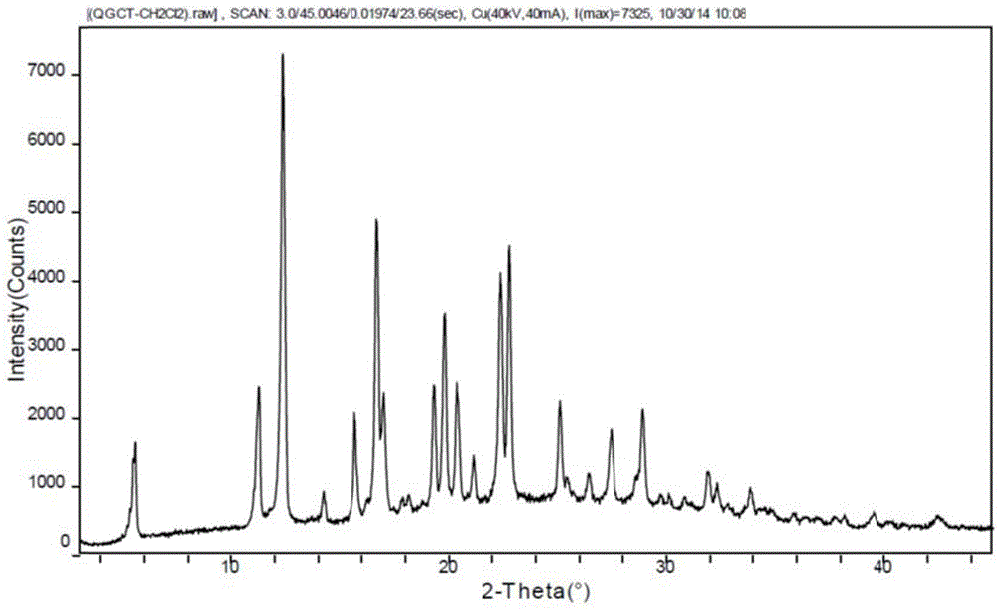
New crystal form and preparation method of highly pure trelagliptin
A high-purity, crystalline technology, applied in organic chemistry and other directions, can solve the problems of undisclosed impact on the quality of the final product, difficult industrial production of purification methods, and limited production capacity improvement, and achieves low-cost, easy recovery, and improved production efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1, the synthesis of compound trexagliptin:
[0068] Compound 1 (10g, 34mmol), (R)-3-aminopiperidine dihydrochloride (8.0g, 47mmol) and sodium bicarbonate (13g, 154mmol) were stirred at reflux in ethanol (200ml) for 3 hours; filtered while hot , the filter cake was washed with ethanol (50ml), and a large amount of solids were precipitated from the filtrate, and the filtrate was stirred for 1 hour in an ice bath. The solid was filtered out, and the solid was recrystallized from ethanol to obtain 10 g of high-purity trexagliptin crystals (yield: 82.1%).
Embodiment 2
[0069] Embodiment 2, the synthesis of compound Trexagliptin:
[0070]Compound 4 (10g, 34mmol), (R)-3-aminopiperidine dihydrochloride (8.0g, 4.7mmol) and sodium bicarbonate (13g, 154mmol) were stirred under reflux in isopropanol (250ml) for 2.5 hours; Filtration while hot, the filter cake was washed with Virahol alcohol (50ml), the filtrate was stirred in an ice bath for 1 hour, and the solid was filtered out, and the solid was recrystallized with Virahol to obtain 10.4g of high-purity Trexagliptin crystals (yield: 85.4%).
Embodiment 3
[0071] Embodiment 3, the synthesis of compound trexagliptin:
[0072] Compound 4 (10g, 34mmol), (R)-3-aminopiperidine dihydrochloride (8.0g, 47mmol) and potassium bicarbonate (21g, 152mmol) were stirred at reflux in ethanol (200ml) for 3.5 hours; filtered while hot , the filter cake was washed with ethanol (50ml), the filtrate was stirred in an ice bath for 1 hour, the solid was filtered off, and the solid was recrystallized with isopropanol to obtain 9.7g of high-purity trexagliptin crystals (80.2% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com