Method for determining related substances in Trelagliptin tablets
A technology related to substances and tablets, applied in the field of analytical chemistry, can solve the problem of impossible to completely control the quality of this product, and achieve the effect of solving the problem of separation and determination and controllable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Instruments and experimental conditions:
[0038] High performance liquid chromatography: Shimadzu LC-16,
[0039] Chromatographic column: XselectC18 (Waters, 150×4.6mm, 3.5um),
[0040] Mobile phase A is phosphoric acid aqueous solution, pH=2.0, mobile phase B is acetonitrile; linear gradient elution according to Table 2.
[0041]
[0042]
[0043] The flow rate is 1.2mL / min,
[0044] Column temperature 35°C,
[0045] Detection wavelength 224nm,
[0046] The injection volume was 10 μl.
[0047] Experimental steps:
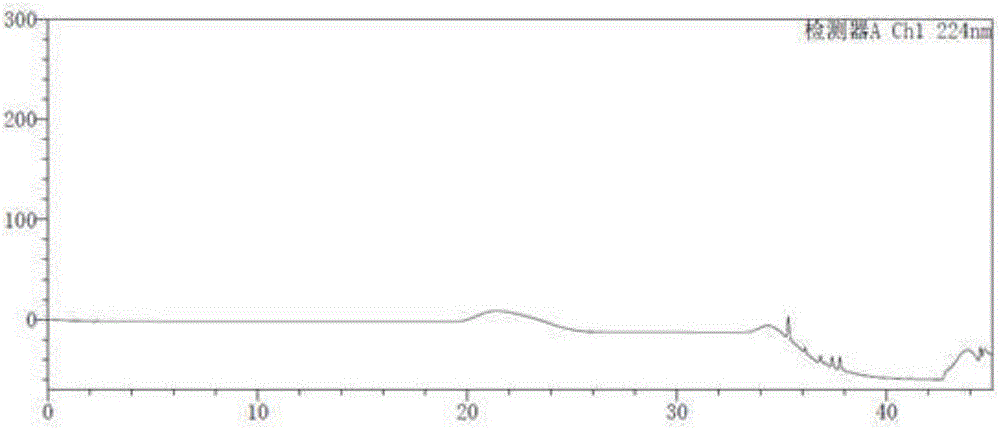
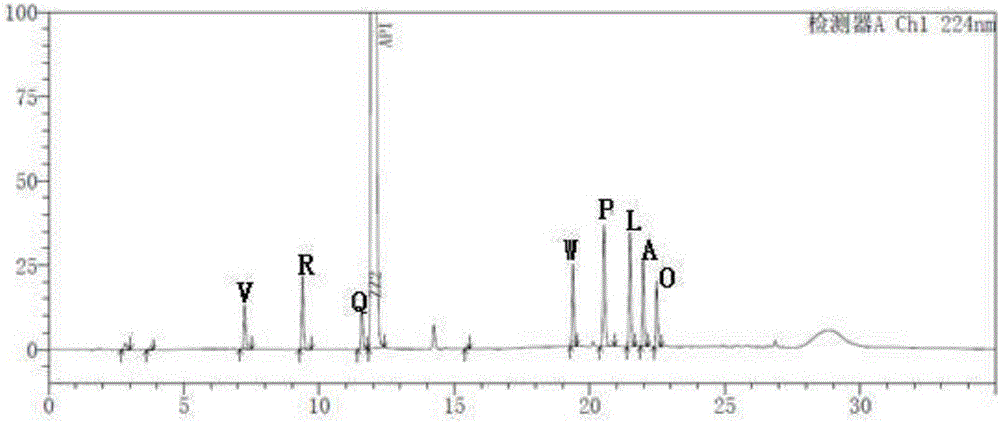
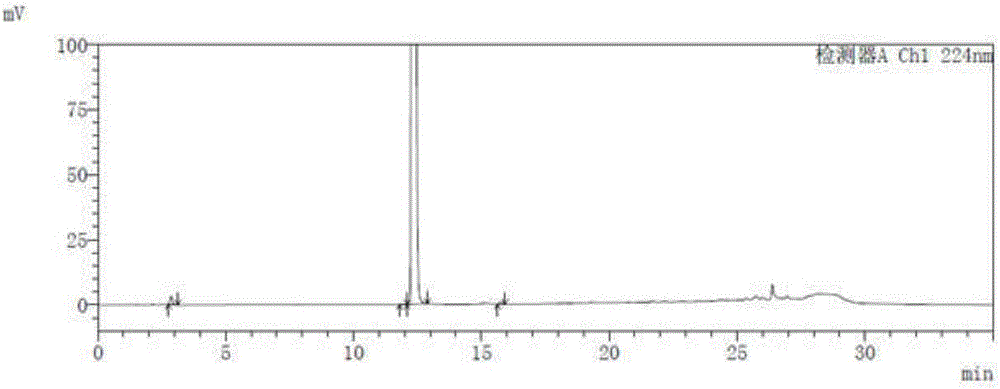
[0048] Take the blank solution and the impurity reference substance solution respectively, carry out high-performance liquid chromatography analysis according to the above experimental conditions, and record the chromatograms. The results are shown in the attached figure 1 And attached figure 2 , API in the figure represents Trexagliptin.
[0049] figure 2 In the middle is the chromatogram corresponding to trexagliptin and each known impurit...
Embodiment 2
[0051] Instruments and experimental conditions:
[0052] High performance liquid chromatography: Shimadzu LC-16,
[0053] Chromatographic column: XselectC18 (Waters, 150×4.6mm, 3.5um),
[0054] Mobile phase A is phosphoric acid aqueous solution, pH 2.0, mobile phase B is acetonitrile; linear gradient elution according to Table 3.
[0055]
[0056]
[0057] The flow rate is 1.2mL / min,
[0058] Column temperature 35°C,
[0059] Detection wavelength 224nm,
[0060] The injection volume was 10 μl.
[0061] Experimental steps:
[0062] Get Trexagliptin tablet sample powder 49.42mg (containing Trexagliptin 24.99mg), put in the 50ml measuring bottle, be 10% acetonitrile dissolving sample with volume percentage, be mixed with every 1mL containing Trexagliptin 0.988mg sample solution.
[0063] Get impurity reference substance solution and trexagliptin tablet sample solution respectively, carry out high performance liquid chromatography analysis by above-mentioned experimental...
Embodiment 3
[0067] Instruments and experimental conditions:
[0068] High performance liquid chromatography: Shimadzu LC-16,
[0069] Chromatographic column: CortecsC18 (Waters, 150×4.6mm, 2.7um),
[0070] Mobile phase A is phosphoric acid aqueous solution, pH=2.0, mobile phase B is acetonitrile solution; linear gradient elution according to Table 5.
[0071]
[0072]
[0073] The flow rate is 1.2mL / min
[0074] Column temperature 35°C
[0075] Detection wavelength 224nm
[0076] The injection volume was 10 μl.
[0077] Experimental steps:
[0078] Take trexagliptin tablet sample powder 50.68mg (containing trexagliptin 25.02mg), put in a 50ml measuring bottle, dissolve the sample with 10% acetonitrile, and be prepared into a sample solution containing trexagliptin 1.01mg per 1mL.
[0079] Take impurity reference substance solution and trexagliptin tablet sample solution respectively, carry out high performance liquid chromatography analysis according to above-mentioned experime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com