Alogliptin-metformin sustained-release tablet and preparation method thereof
A technology of metformin and metformin hydrochloride, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., to achieve the effect of stable and slow release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: specification 6.25mg alogliptin (equivalent to 8.5mg alogliptin benzoate) and 500mg metformin hydrochloride
[0098] Compound Alogliptin Metformin Sustained Release Tablets (Formulation)
[0099]
[0100] Preparation:
[0101] 1. Drug loading: Dissolve metformin hydrochloride in purified water with a solubility of 300mg / ml; add the adsorbent silicon dioxide to the above metformin hydrochloride solution and stir for 24 hours to make the inner and outer surfaces of the silicon dioxide completely covered by the metformin hydrochloride solution infiltration.
[0102] 2. Curing: Add polyvinylpyrrolidone K30, the binder part of metformin, to the suspension system prepared in step 1 to prepare a suspension with a solid content of about 30%, spray dry it with LPG-10 spray drier, and feed it into the The temperature is 160° C., and the outlet air temperature is 80° C. to obtain solid drug-containing granules.
[0103] 3. Grain sizing: In step 2, the granules ...
Embodiment 2
[0108] Embodiment 2: specification 6.25mg alogliptin (equivalent to 8.5mg alogliptin benzoate) and 1000mg metformin hydrochloride
[0109] Compound Alogliptin Metformin Sustained Release Tablets (Formulation)
[0110] Raw materials
Formula amount (g)
Metformin Hydrochloride Extended Release Fraction
1000
Silica (Aerosil300)
145
Hypromellose K100M
220
30
8
Subtotal
1403
Alogliptin immediate release portion
8.5
Polyoxyethylene Hydrogenated Castor Oil
8.5
Hypromellose E5
4
4
total
1428
Make 1000 capsules
[0111] Preparation:
[0112] 1. Drug loading: Dissolve metformin hydrochloride in purified water with a solubility of 300mg / ml; add the adsorbent silicon dioxide to the above metformin hydrochlori...
Embodiment 3
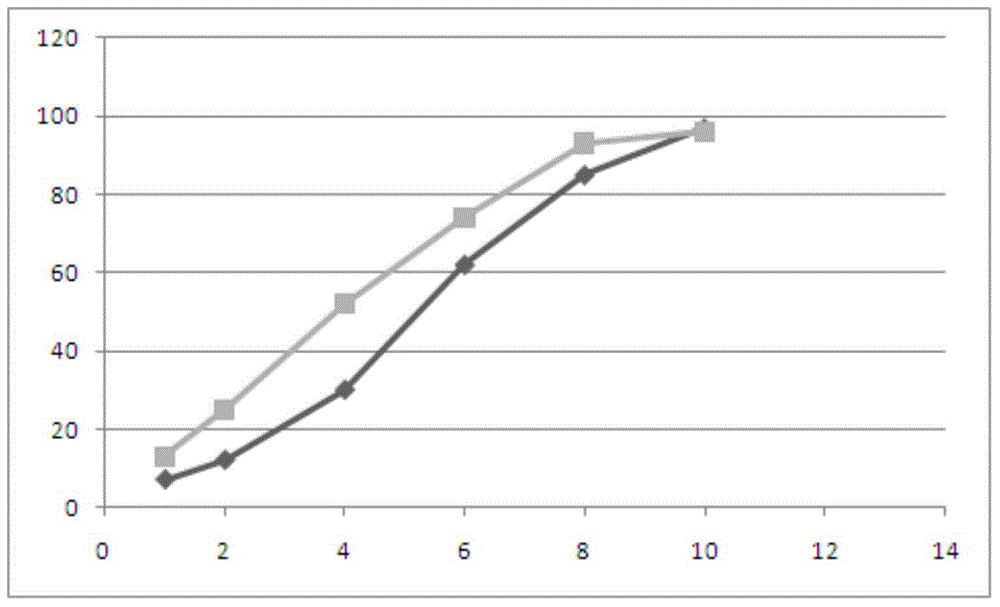
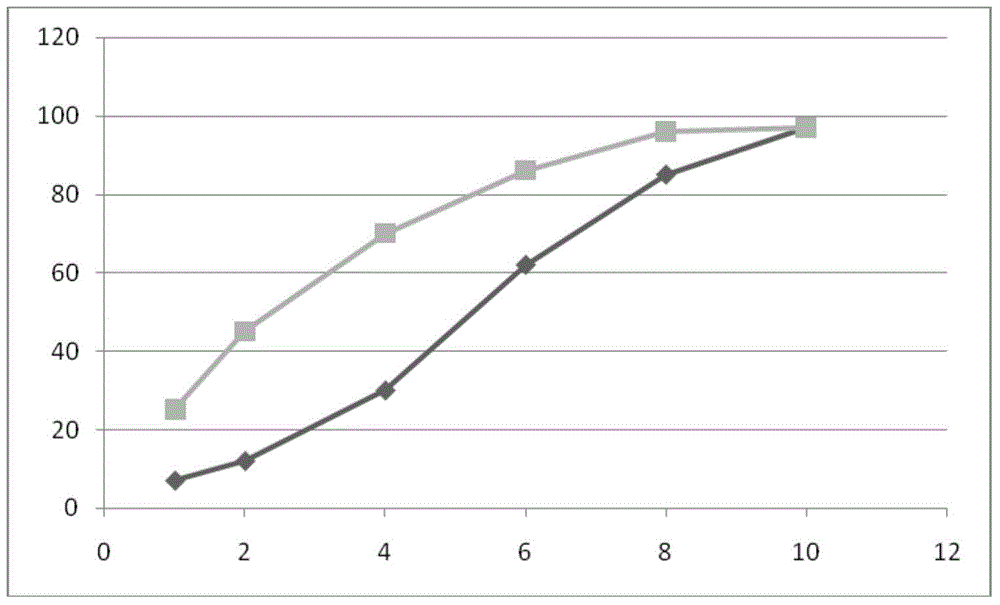
[0120] The compound alogliptin metformin sustained-release tablet prepared in embodiment 1 and embodiment 2 adopts Chinese Pharmacopoeia 2005 edition dissolution rate detection method to measure release rate, condition is paddle method, rotating speed is 50 revs / min, and dissolution medium is 37 ℃ 900 ml of pH 6.8 phosphate buffer. The comparative results of the release rates of Example 1 and Example 2 are as follows.
[0121] Metformin release:
[0122] time point (hour)
1
2
4
6
8
10
Example 1 (%)
7
12
30
62
85
97
Example 2 (%)
13
25
52
74
93
96
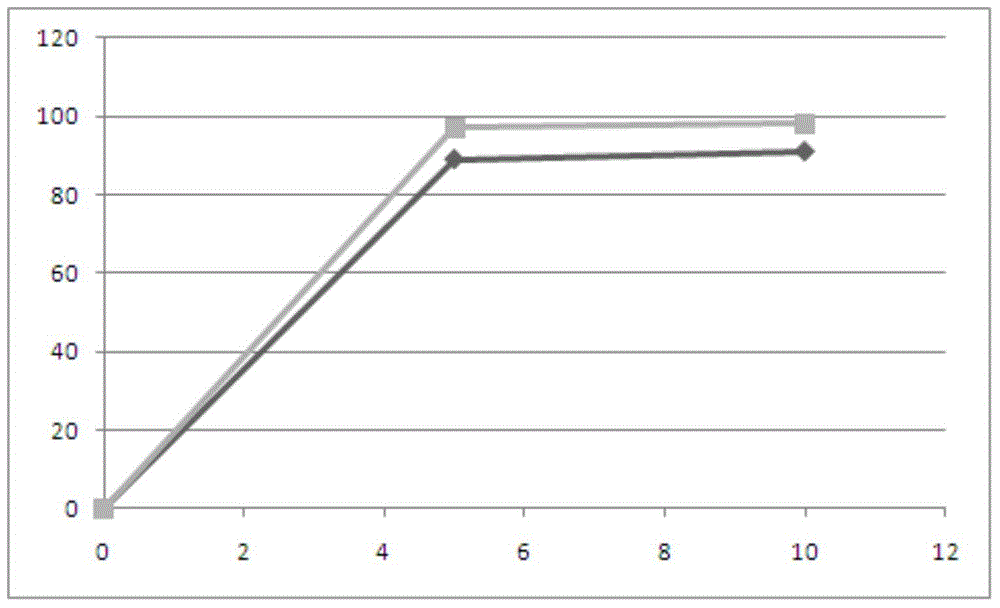
[0123] Alogliptin Release Rate:
[0124] time point (minute)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com