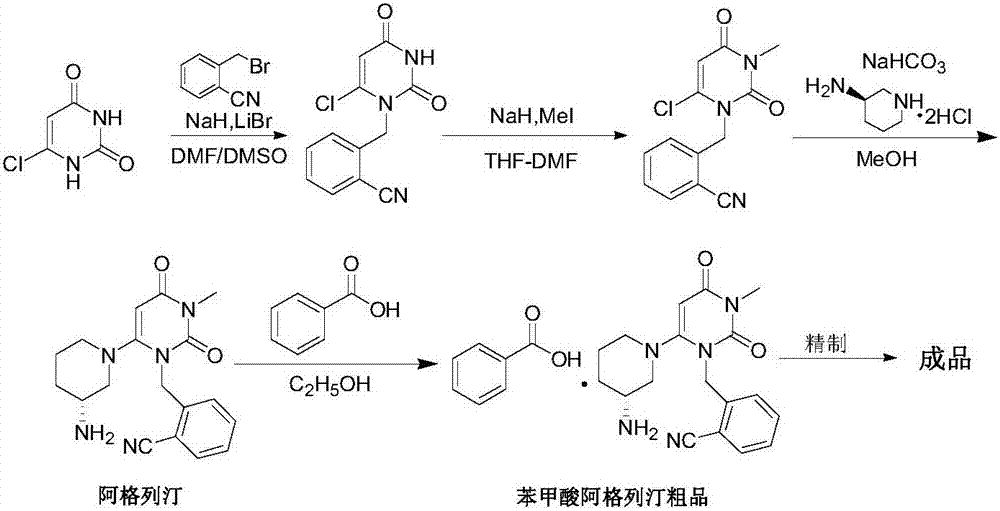
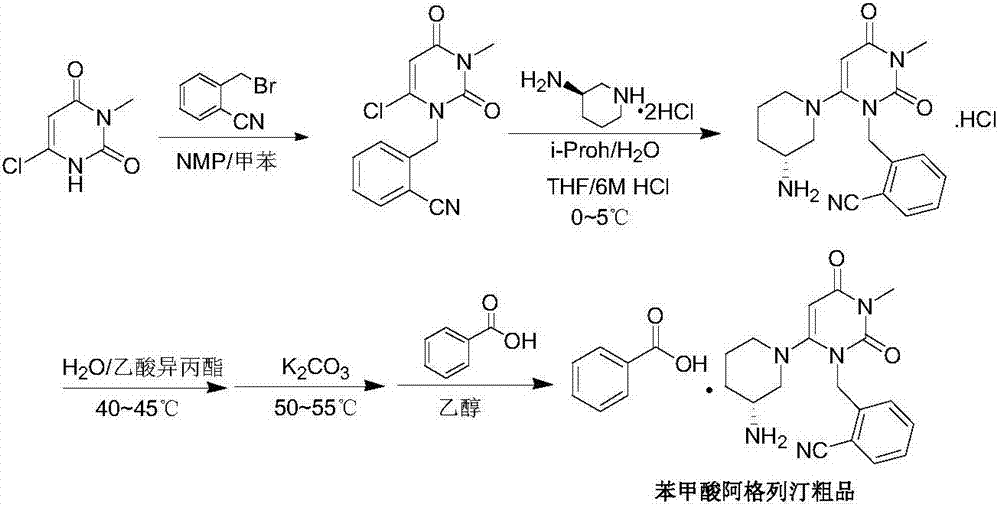
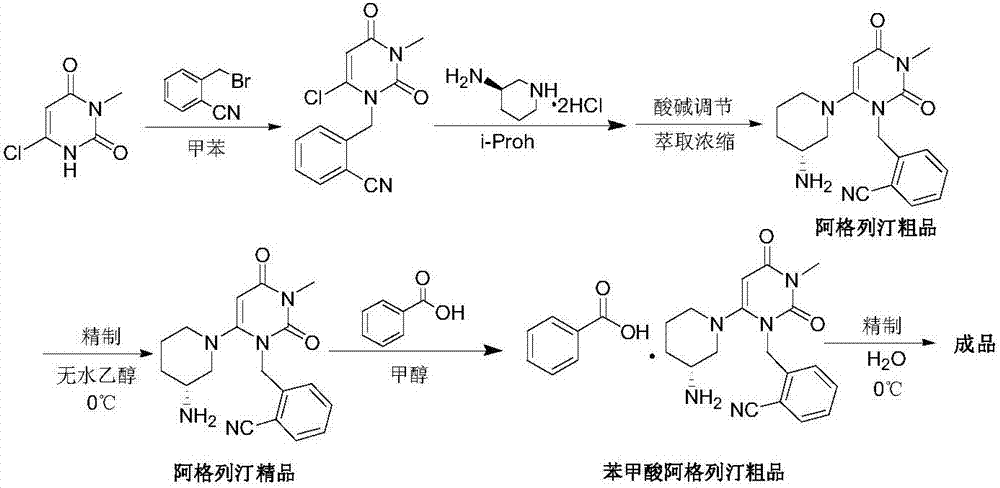
A preparing method for alogliptin benzoate
A technology of benzoic acid and methyl group, applied in the field of preparation of improved processes, can solve the problems of low total yield, high industrialization cost, unfavorable recovery and mechanical application, etc., and achieves the effects of improving yield and purity, being easy to industrialize amplification, and being environmentally friendly in process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)pyrimidinyl)methyl]benzonitrile (intermediate 1) preparation
[0060] In a 2L reactor with mechanical stirring and condenser, add 6-chloro-3-methyluracil (80g, 498.3mmol), 2-cyanobenzyl bromide (97.7g, 498.3mmol), ethyl acetate (600g ), diisopropylethylamine (128.8g, 996.6mmol) was reacted at about 70-75°C for 4 hours, cooled to 0-5°C, stirred for 5 hours, suction filtered, rinsed with a small amount of cold ethyl acetate, and obtained Add 800g of purified water to the wet product, stir wash at about 25°C for 20 minutes, filter with suction, and dry the wet product at 50°C to obtain 125.5g of off-white solid. Yield: 91.3%, HPLC purity: 99.76%.
Embodiment 2
[0061] Example 2 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)pyrimidinyl)methyl]benzonitrile (intermediate 1) preparation
[0062] In a 2L reactor with mechanical stirring and condenser, add 6-chloro-3-methyluracil (80g, 498.3mmol), 2-cyanobenzyl bromide (97.7g, 498.3mmol), propyl acetate (480g ), diisopropylethylamine (102g, 789.2mmol) was reacted at about 70-75°C for 4 hours, cooled to 5-10°C, stirred for 5 hours, filtered with suction, rinsed with a small amount of cold propyl acetate, and obtained wet Add 800g of purified water to the product, stir wash at about 25°C for 20 minutes, filter with suction, and dry the wet product at 50°C to obtain 128.1g of off-white solid. Yield: 93.2%, HPLC purity: 99.51%.
Embodiment 3
[0063] Example 3 2-[(6-chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)pyrimidinyl)methyl]benzonitrile (intermediate 1) Scale up preparation
[0064] In a 200L reactor with mechanical stirring and condenser, add 6-chloro-3-methyluracil (8kg, 49.83mol), 2-cyanobenzyl bromide (10.58kg, 53.97mol), ethyl acetate (6.4 kg) and diisopropylethylamine (10.2kg, 78.92mol) were reacted at about 70-75°C for 4 hours, cooled to -5-0°C, stirred for 3 hours, filtered with suction, rinsed with a small amount of cold ethyl acetate , to obtain a wet product, add 800g of purified water, stir and wash at about 25°C for 20min under temperature control, filter with suction, and dry the wet product at 50°C to obtain 12.38kg of an off-white solid. Yield: 90.1%, HPLC purity: 99.91%. IR (cm -1 ): 3095, 2228, 1655, 1604, 1441, 1398, 1203, 1093, 981, 766 and 522. NMR 1 H-NMR (400MHz, CDCl 3 ): δH 7.75(1H,d,br), 7.63(1H,td), 7.44(1H,t), 7.24(1H,d), 6.01(1H,d), 5.51(2H,s), 3.38(3H ,s). ESI-MS(m / z):276[M+H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com