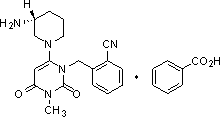
Industrial production method of Alogliptin benzoate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
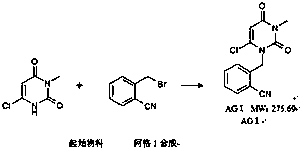
[0050] The industrial production method of alogliptin benzoate of the present invention comprises three major steps, the first step prepares alogliptin intermediate AGⅠ, the second step prepares alogliptin intermediate AGII, and the third step prepares alogliptin benzoate Ting; the specific method is as follows.
[0051] The first step is to prepare the alogliptin intermediate AGI.
[0052](1) Add 24.5kg of N-methylpyrrolidone, 8.0kg of 6-chloro-3-methyluracil and 7.18kg of N,N-diisopropylethylamine into a 300L glass-lined reactor, and stir until dissolved.
[0053] (2) Add 8.16 kg of 2-cyanobenzyl bromide into the reaction kettle, and stir to dissolve.
[0054] (3) Heat the reaction kettle to 60-65°C, stir and keep warm for 3 hours; start TLC monitoring after 3 hours until the reaction ends, that is, the raw material point of 6-chloro-3-methyluracil basically disappears; take samples for monitoring every 0.5 hours.
[0055] The reaction endpoint monitoring method is as foll...
Embodiment 2
[0108] The first step is to prepare the alogliptin intermediate AGI.
[0109] (1) Add 36.75kg of N-methylpyrrolidone, 12.0kg of 6-chloro-3-methyluracil and 10.77kg of N,N-diisopropylethylamine into a 300L glass-lined reactor, and stir until dissolved.
[0110] (2) Add 12.24 kg of 2-cyanobenzyl bromide into the reaction kettle, stir to dissolve.
[0111] (3) Heat the reaction kettle to 60-65°C, stir and keep warm for 3 hours; start TLC monitoring after 3 hours until the reaction ends, that is, the raw material point of 6-chloro-3-methyluracil basically disappears; take samples for monitoring every 0.5 hours.
[0112] The reaction endpoint monitoring method is as follows.
[0113] ①Preparation of the test solution: take about 0.2ml of the reaction solution, add 2ml of N-methylpyrrolidone to dilute, and obtain the product.
[0114] ②Preparation of the control solution: Take about 2mg of 6-chloro-3-methyluracil, add 2ml of N-methylpyrrolidone to dissolve it.
[0115] ③ Chromato...
Embodiment 3
[0157] The first step is to prepare the alogliptin intermediate AGI.
[0158] (1) Add 29.0kg of N-methylpyrrolidone, 16.0kg of 6-chloro-3-methyluracil and 17.36kg of N,N-diisopropylethylamine into a 300L glass-lined reactor, and stir until dissolved.
[0159] (2) Add 16.32 kg of 2-cyanobenzyl bromide into the reaction kettle, and stir to dissolve.
[0160] (3) Heat the reaction kettle to 60-65°C, stir and keep warm for 3 hours; start TLC monitoring after 3 hours until the reaction ends, that is, the raw material point of 6-chloro-3-methyluracil basically disappears; take samples for monitoring every 0.5 hours.
[0161] The reaction endpoint monitoring method is as follows.
[0162] ①Preparation of the test solution: take about 0.2ml of the reaction solution, add 2ml of N-methylpyrrolidone to dilute, and obtain the product.
[0163] ②Preparation of the control solution: Take about 2mg of 6-chloro-3-methyluracil, add 2ml of N-methylpyrrolidone to dissolve it.
[0164] ③ Chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com