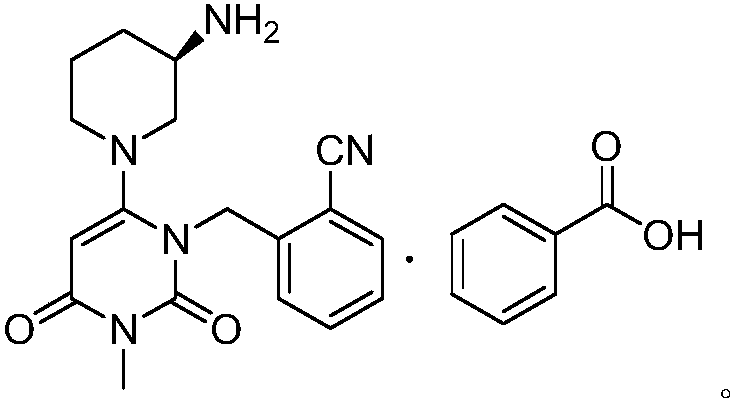
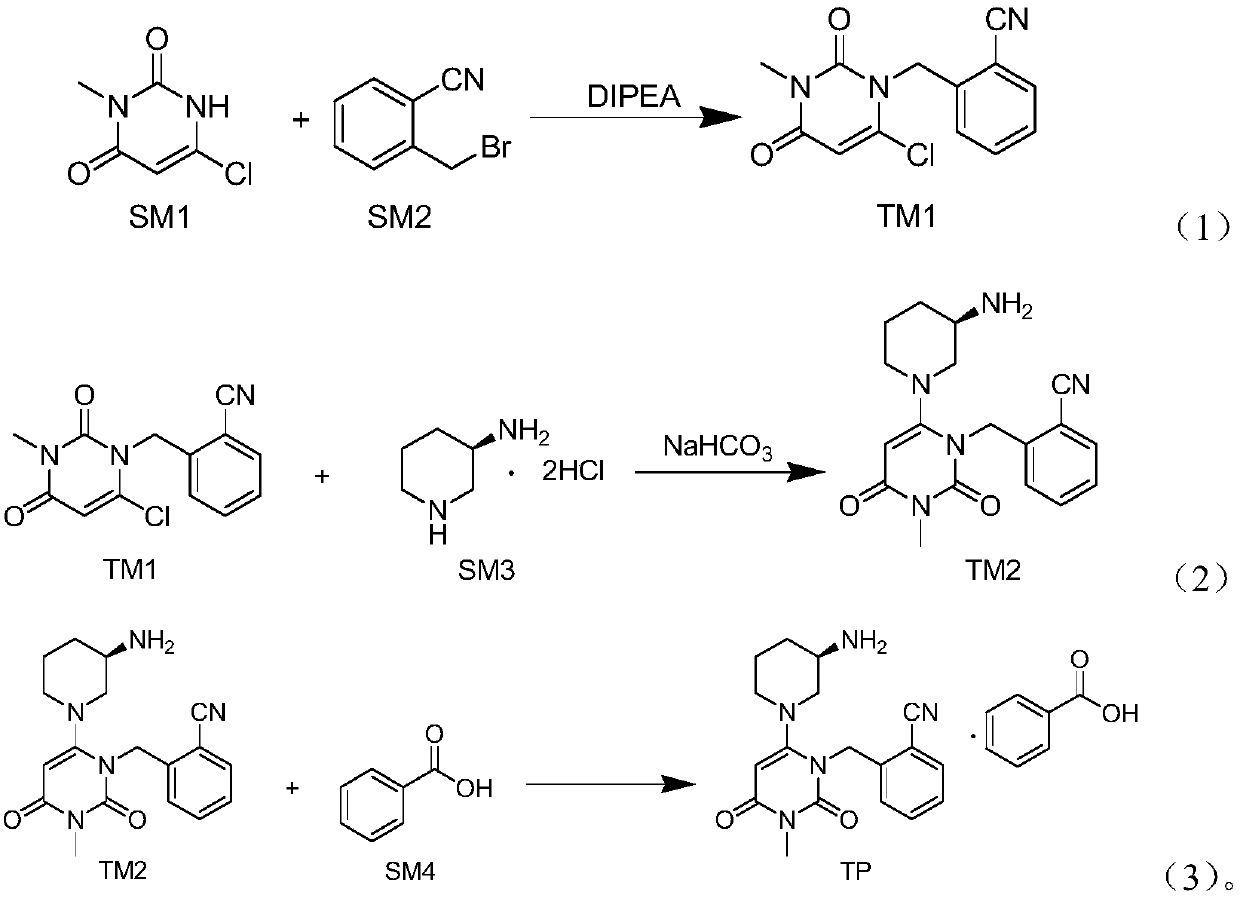
Preparation method of alogliptin benzoate
A technology of benzoic acid and methyl, which is applied in the field of preparation of alogliptin benzoate, can solve the problems affecting the purity and yield of the target product, and achieve the effects of reducing difficulty, reducing losses, and reducing the generation of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) At 25°C, add 10.0g (62.3mmol) SM1, 40mL N-methylpyrrolidone, and 12.1g (93.6mmol) diisopropylethylamine into a 500mL three-necked flask in sequence, and then pour it into the reaction flask under stirring 40 mL of toluene solution in which 13.4 g (68.4 mmol) of SM2 was dissolved was added dropwise. After the addition was completed, the temperature was raised to 70° C., and the reaction was carried out for 1.5 hours. The reaction system was lowered to room temperature, 40 mL of water was added thereto, stirred at 25° C. for 30 minutes, cooled to 0° C., stirred for 1 hour, and suction filtered. The filter cake was rinsed with 10 mL of isopropanol and then dried to obtain 15.5 g of off-white solid with a yield of 90.2%. 1 HNMR (400MHz, DMSO-d 6 )δppm 7.90(d, J=7.6Hz, 1H), 7.71(t, J=7.8Hz, 1H), 7.52(t, J=7.8Hz, 1H), 7.42(d, J=8.0Hz, 1H), 6.24(s,1H), 5.39(s,2H), 3.19(s,3H).
[0028] (2) Add 11.3g (65.3mmol) SM3, 22.5mL water, and 39.5g (470.2mmol) sodium bicarbonate t...
Embodiment 2
[0031] (1) At 25°C, add 10.0g (62.3mmol) SM1, 48mL N-methylpyrrolidone, and 16.1g (124.6mmol) diisopropylethylamine into a 500mL three-necked flask in sequence, and then pour it into the reaction flask under stirring 40 mL of toluene solution in which 18.3 g (93.5 mmol) of SM2 was dissolved was added dropwise. After the dropwise addition was completed, the temperature was raised to 73° C. and reacted for 3 hours. The reaction system was lowered to room temperature, 40 mL of water was added thereto, stirred at 25° C. for 30 minutes, cooled to 0° C., stirred for 1 hour, and suction filtered. The filter cake was rinsed with 10 mL of isopropanol and then dried to obtain 14.5 g of off-white solid with a yield of 84.4%.
[0032] (2) Add 11.3g (65.3mmol) SM3, 30mL water, and 54.9g (653.0mmol) sodium bicarbonate to a 500mL reaction flask in sequence at 25°C, and stir for 30 minutes at this temperature. At 25°C, 75 mL of tetrahydrofuran and 15.0 g (54.4 mmol) of TM1 were added to the ...
Embodiment 3
[0035] (1) At 25°C, add 10.0g (62.3mmol) SM1, 32mL N-methylpyrrolidone, and 8.1g (62.3mmol) diisopropylethylamine into a 500mL three-necked flask in sequence, and then add 40 mL of toluene solution in which 12.2 g (62.3 mmol) of SM2 was dissolved was added dropwise. After the dropwise addition was completed, the temperature was raised to 68° C., and the reaction was carried out for 1 hour. The reaction system was lowered to room temperature, 40 mL of water was added thereto, stirred at 25° C. for 30 minutes, cooled to 0° C., stirred for 1 hour, and suction filtered. The filter cake was rinsed with 10 mL of isopropanol and then dried to obtain 11.6 g of off-white solid with a yield of 67.5%.
[0036] (2) Add 11.3g (65.3mmol) SM3, 15mL water, and 22.8g (272.0mmol) sodium bicarbonate to a 500mL reaction flask in sequence at 25°C, and stir for 30 minutes at this temperature. At 25°C, 75 mL of acetonitrile and 15.0 g (54.4 mmol) of TM1 were added to the reaction flask. The temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com