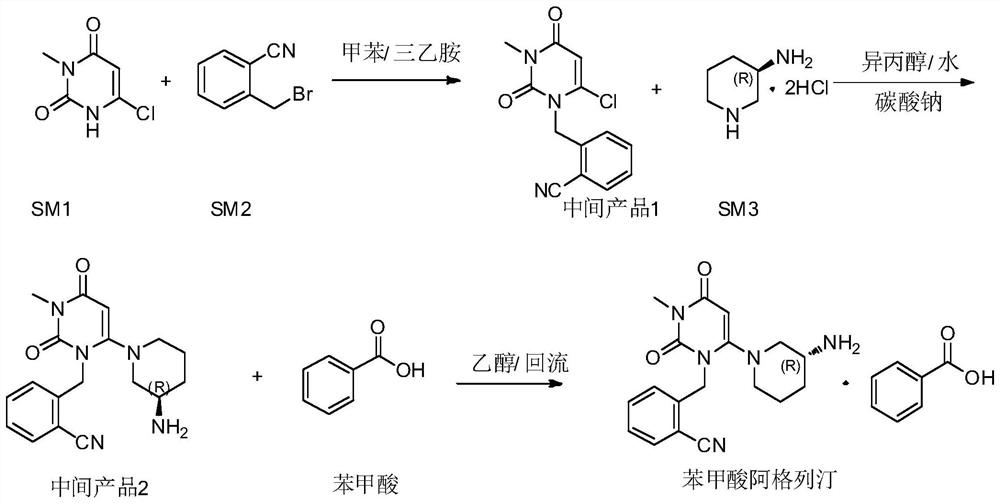
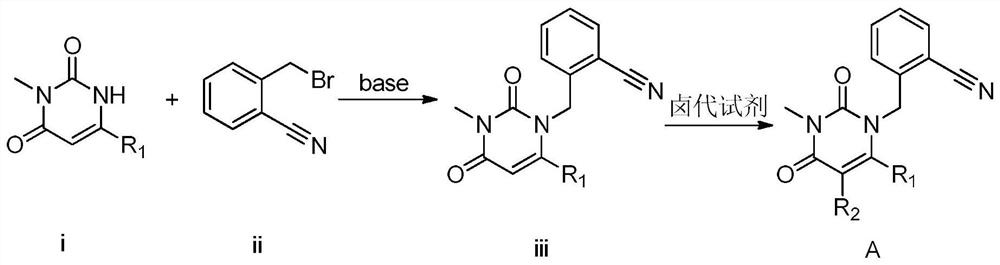
3, 4-dihydropyrimidine benzonitrile derivative and preparation method and application thereof
A technology of dihydropyrimidine and benzonitrile, which is applied in the field of chemical medicine and can solve problems such as reducing the content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
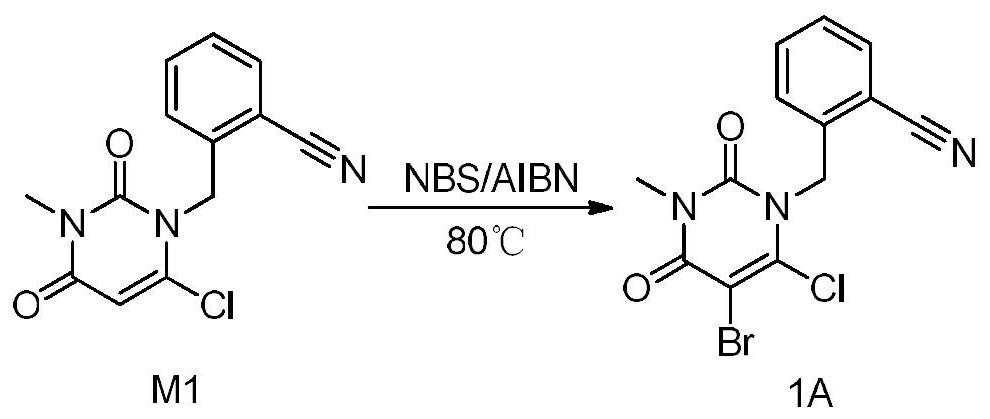
[0090] Embodiment 1 Preparation of compound of formula (1A): 2-((5-bromo-6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl ) methyl) benzonitrile
[0091] The synthetic route of formula (1A) compound sees image 3 . The specific method is as follows:
[0092] Put dichloroethane (45ml) and compound M1 (3.00, 10.9mmol) into a three-necked flask, add NBS (2.33g, 13.1mmol) and AIBN (0.09g, 0.5mmol) under stirring, turn on the heating, and the reaction solution Raise the temperature to 80°C for 3 hours, turn off the heating, and cool down to 20°C in an ice-water bath. Add 30ml of water to the reaction solution, stir and wash, collect the lower organic phase, and continue to wash with 15ml of 5% NaHCO 3 The solution was washed twice and once with 15 ml of saturated brine, and the lower organic phase was collected and dried by adding 3 g of anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain off-white solid powder. ...
Embodiment 2
[0094] Example 2 Preparation of compound of formula (2A): 2-((5-bromo-6-methoxy-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H) -yl)methyl)benzonitrile
[0095] The synthetic route of formula (2A) compound sees Figure 4 . The specific method is as follows:
[0096] Add compound 1A (5g, 14.1mmol) and 40ml methanol into a three-necked flask, stir to dissolve and add K 2 CO 3 (3.90 g, 28.2 mmol). Turn on the heating, and keep the reaction at 65°C for 6h. The reaction solution was concentrated to dryness under reduced pressure, then 40ml of water and 30ml of dichloromethane were added, the organic phase was collected after stirring, the aqueous phase was extracted twice with 20ml of dichloromethane, the organic phases were combined, and dried by adding 5g of anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain an off-white solid powder, which was beaten with 20 ml MTBE at room temperature for 2 h. After filtra...
Embodiment 3
[0099] Example 3 Preparation of compound of formula (3A): (R)-2-((6-(3-aminopiperidin-1-yl)-5-bromo-3-methyl-2,4-dioxo- 3,4-Dihydropyrimidin-1(2H)-yl)methyl)benzonitrile
[0100] The synthetic route of formula (3A) compound sees Figure 5 . The specific method is as follows:
[0101] Put dichloroethane (45ml) and compound M2 (5.00, 14.7mmol) into a three-necked flask, add NBS (3.70g, 20.8mmol) and AIBN (0.09g, 0.7mmol) under stirring, turn on the heating, and the reaction solution Raise the temperature to 80°C for 4 hours, turn off the heating, and cool down to 20°C in an ice-water bath. Add 30ml of water to the reaction solution, stir and wash, collect the lower organic phase, and continue to wash with 15ml of 5% NaHCO 3 The solution was washed twice and once with 15 ml of saturated brine, and the lower organic phase was collected and dried by adding 3 g of anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain off-whit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com