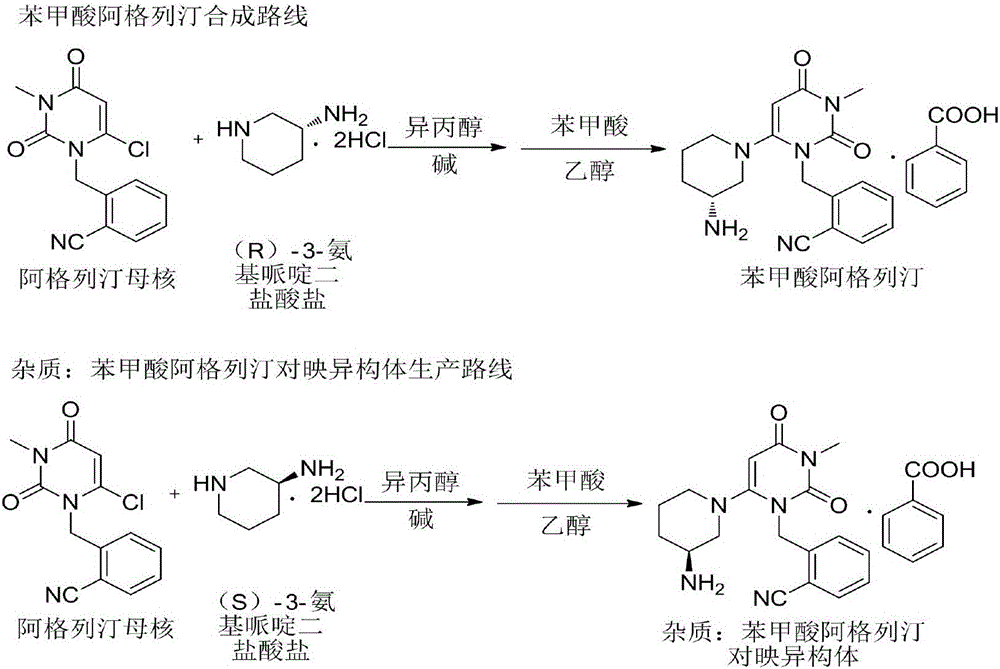
Method for refining alogliptin benzoate
A purification method and technology of benzoic acid, applied in the direction of organic chemistry and the like, can solve the problems such as inability to remove alogliptin benzoate isomers, and achieve the effect of ensuring safety and effectiveness and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A method for refining alogliptin benzoate, comprising the following steps:
[0038] Put 10 g of alogliptin benzoate crude product and 60 mL of acetone into the reaction flask, raise the temperature to 45-55° C., and stir until all the solids are dissolved. Add 40 mL of 1.5 mol / L d-tartaric acid aqueous solution to the reaction flask, and continue to stir and react at 45-55° C. for 1 h.
[0039] Cool the reaction solution with ice-salt water to 0-5°C, stir and crystallize for 2-3 hours, filter, remove tartaric acid from the solid through subsequent operations, and then salt and crystallize with benzoic acid, filter, and dry the filter cake under reduced pressure to constant weight. Obtain 9.42 g of alogliptin benzoate.
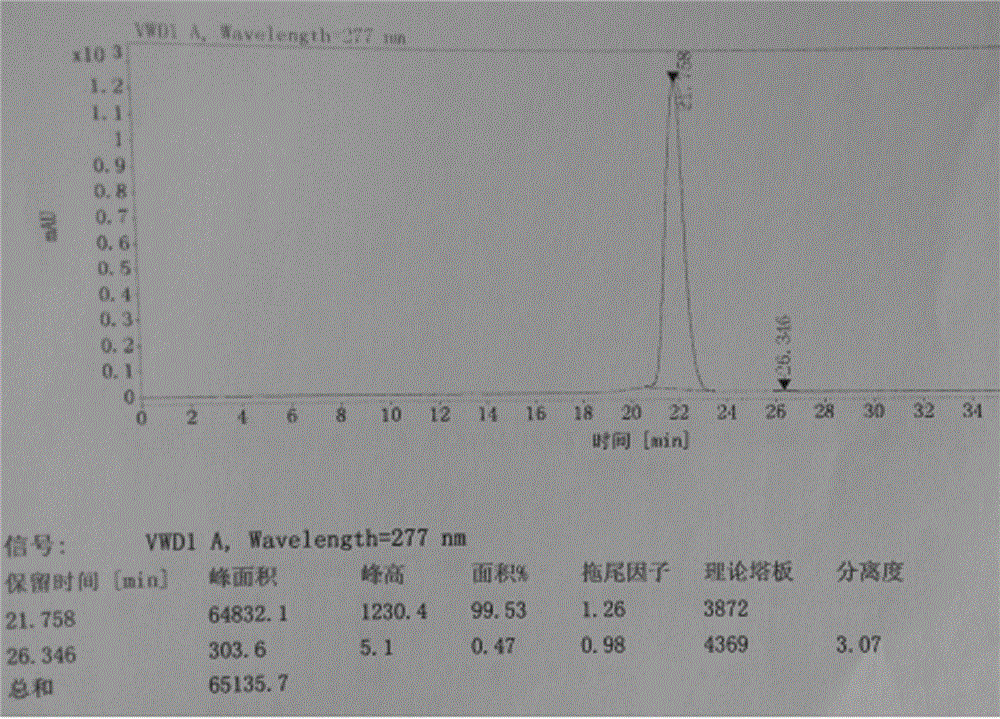
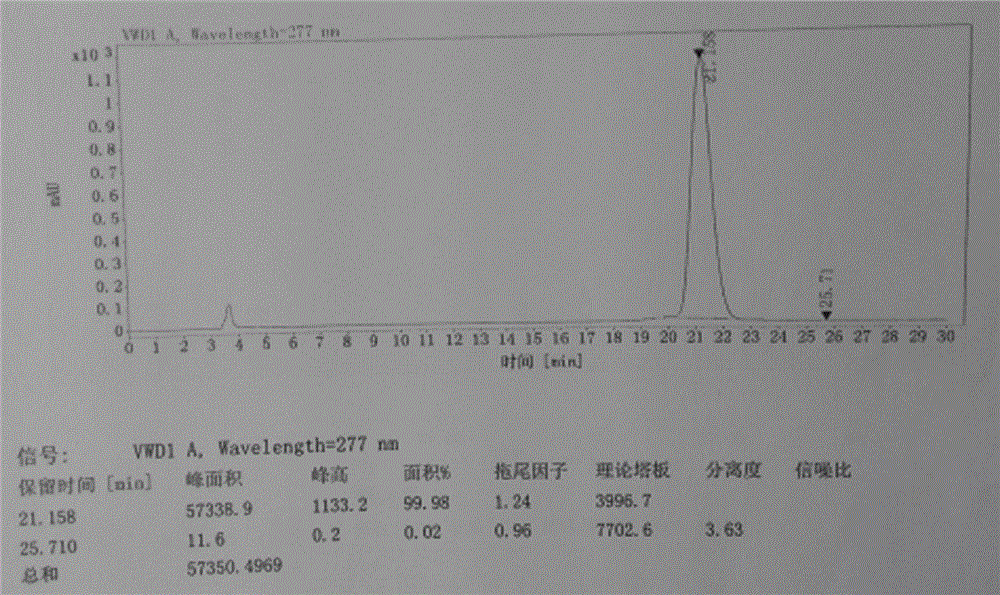
[0040] The refined alogliptin benzoate is detected by HPLC, and the purity is 99.98%, and the impurity content of the alogliptin benzoate enantiomer is 0.02%. The liquid chromatograms of alogliptin benzoate before and after refining are shown in figur...
Embodiment 2
[0042] Put 10 g of alogliptin benzoate crude product and 60 mL of tetrahydrofuran into a reaction flask, raise the temperature to 40-45° C., and stir until all the solids are dissolved. Add 40 mL of 1.5 mol / L d-tartaric acid aqueous solution to the reaction flask, and continue to stir and react at 40-45° C. for 1 h.
[0043] Cool the reaction solution with ice-salt water to 0-5°C, stir and crystallize for 1 hour, filter, remove tartaric acid through subsequent operations, and then salt and crystallize with benzoic acid, filter, and dry the filter cake under reduced pressure to constant weight to obtain benzoic acid Alogliptin 9.33g.
[0044] The refined alogliptin benzoate is detected by HPLC, and the purity is 99.95%, and the impurity content of the alogliptin benzoate enantiomer is 0.05%.
Embodiment 3
[0046] Put 10 g of alogliptin benzoate crude product and 100 mL of acetonitrile into the reaction flask, raise the temperature to 50-60° C., and stir until all the solids are dissolved. Add 40 mL of 1.5 mol / L d-tartaric acid aqueous solution to the reaction flask, and continue to stir and react at 50-60° C. for 1 h.
[0047]Cool the reaction solution with ice-salt water to 0-5°C, stir and crystallize for 4 hours, filter, remove tartaric acid through subsequent operations, and then salt and crystallize with benzoic acid, filter, and dry the filter cake under reduced pressure to constant weight to obtain benzoic acid Alogliptin 8.98g.
[0048] The refined alogliptin benzoate is detected by HPLC, and the purity is 99.98%, and the impurity content of the alogliptin benzoate enantiomer is 0.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com