Synthetic method of azithromycin impurity F
A technology of azithromycin and synthetic method, applied in the field of chemistry, can solve problems such as low synthetic yield of impurity I, and achieve the effects of stable properties, high reaction efficiency and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
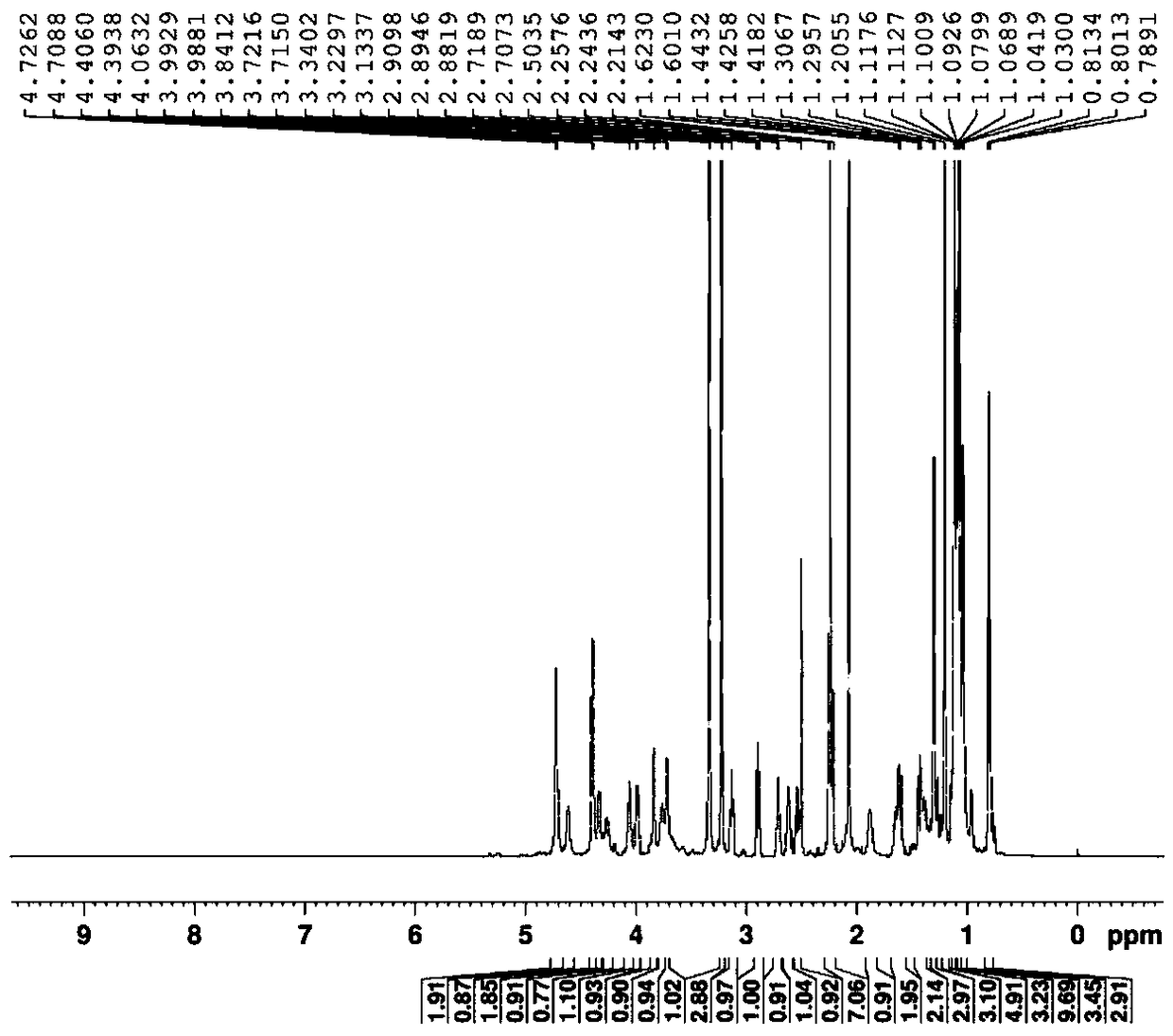
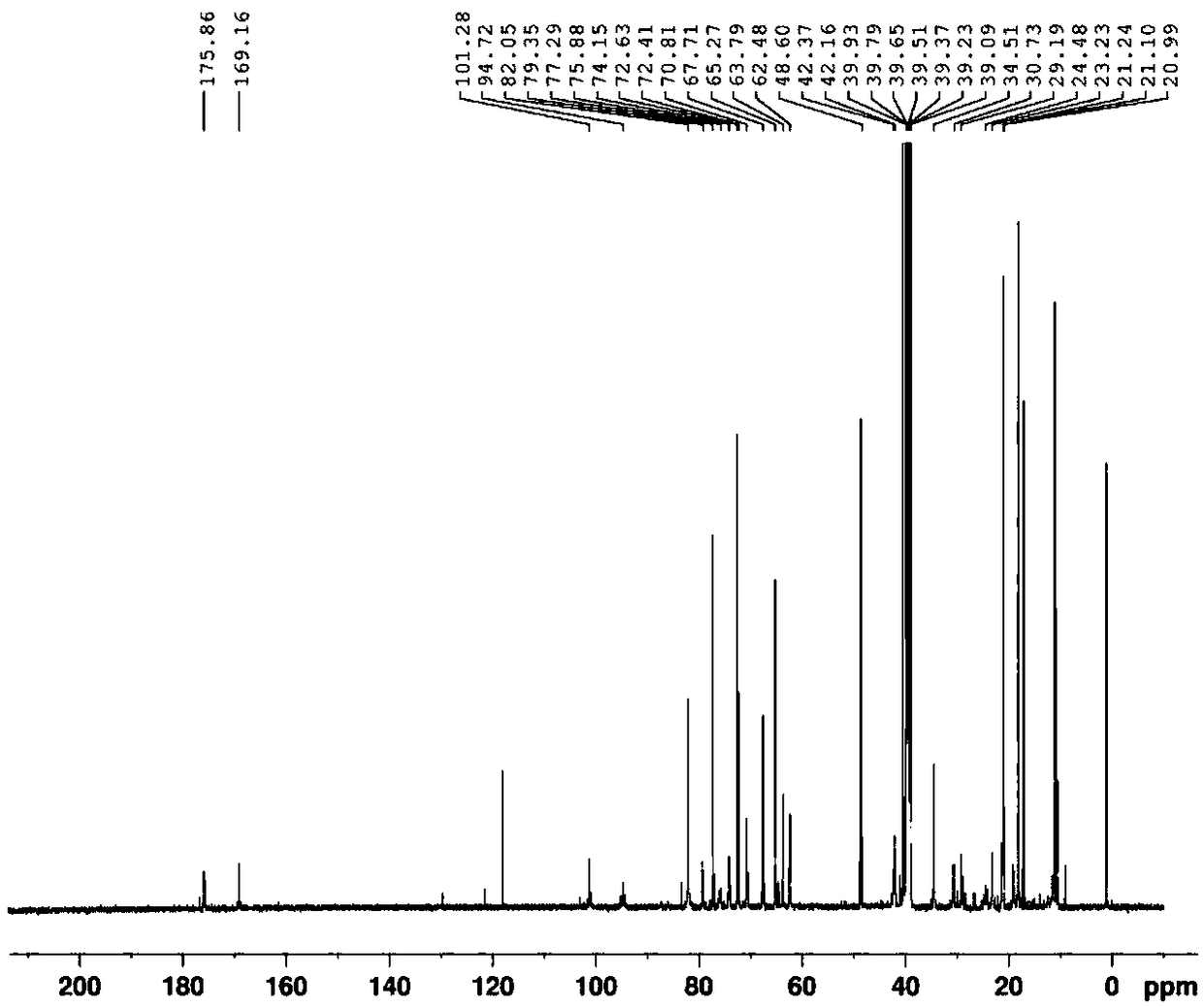
[0036] Example 1 (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl- a-L-nucleo-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3 ,4,6-Trideoxy-3-(N-methylformamido)-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecane-15 Ketone synthesis
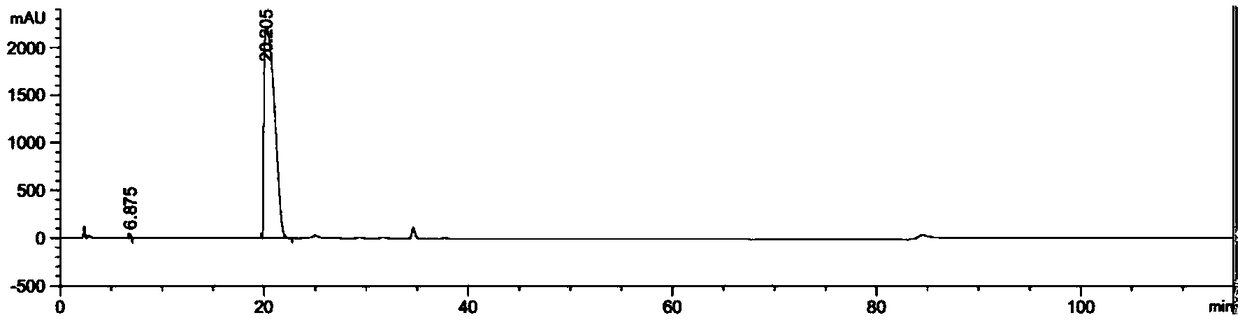
[0037] Add 20g (26mmol) of azithromycin, 400ml of methanol-water (4:1) into a three-necked flask, add 6.6g (52mmol) of iodine, 4g (29mmol) of potassium carbonate, 2.4g (32mmol) of ethyl formate, and stir to reflux Reaction for 5 hours; methanol was recovered by distillation under reduced pressure, washed with water, and suction filtered to obtain 18 g of white solid, yield 90%.
[0038] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-nucleus -hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4, Purification of 6-trideoxy-3-(N-methylformamido)-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one
[00...
Embodiment 2
[0040] Example 2 (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl- a-L-nucleo-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3 ,4,6-Trideoxy-3-(N-methylformamido)-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecane-15 Ketone synthesis
[0041] Add 20g (26mmol) of azithromycin, 200ml of methanol-water (4:1) into a three-necked flask, add 6.6g (52mmol) of iodine, 4g (29mmol) of potassium carbonate, 2.4g (32mmol) of ethyl formate, and stir to reflux Reaction for 5 h; methanol was recovered by distillation under reduced pressure, washed with water, and suction filtered to obtain 15 g of white solid, yield 75%.
[0042] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-nucleus -hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4, Purification of 6-trideoxy-3-(N-methylformamido)-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one
[0043] ...
Embodiment 3
[0044] Example 3 (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl- a-L-nucleo-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3 ,4,6-Trideoxy-3-(N-methylformamido)-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecane-15 Ketone synthesis
[0045] Add 20g (26mmol) of azithromycin, 400ml of methanol-water (4:1) into a three-necked flask, add 6.0g (47mmol) of iodine, 4g (29mmol) of potassium carbonate, 2.4g (32mmol) of ethyl formate, and stir to reflux Reacted for 5 hours; methanol was recovered by distillation under reduced pressure, washed with water, and suction filtered to obtain 17.8 g of white solid, with a yield of 89%.
[0046] (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-a-L-nucleus -hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4, Purification of 6-trideoxy-3-(N-methylformamido)-β-D-xyl-hexapyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com