Method for preparing and refining eplerenone
A technique of refining eplerenone, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as low overall yield, potential safety hazards, and many steps, and achieve the effects of shortening reaction time, saving production costs, and shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of eplerenone crude product
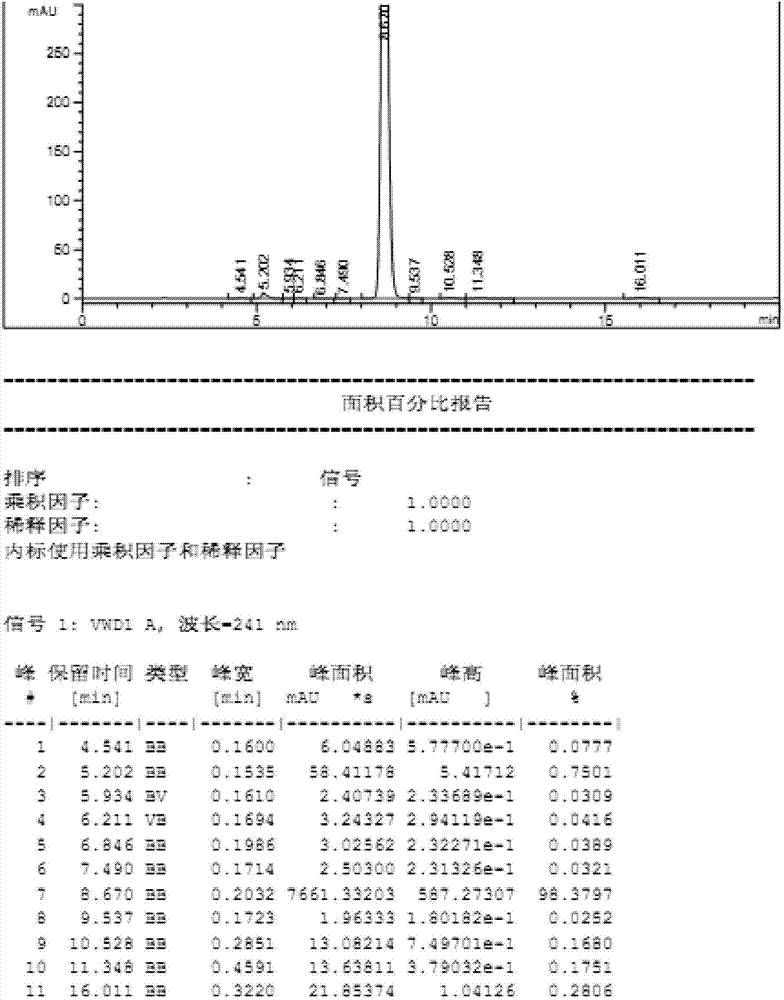
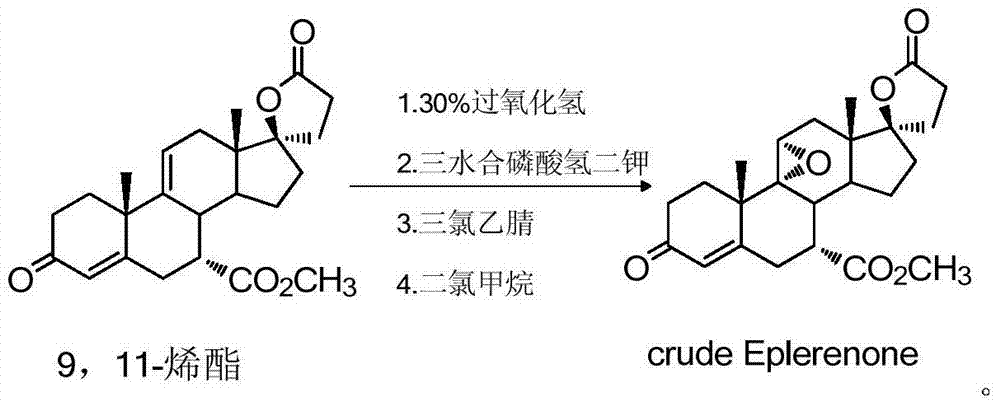
[0042] Add 450 g of 9,11-enyl ester, 180.4 g of dipotassium hydrogen phosphate trihydrate, 366.8 g of trichloroacetamide, and 2700 ml of dichloromethane in sequence in a 5 L reaction flask at room temperature, and start stirring. Under the protection of nitrogen, start to add 30% hydrogen peroxide solution (15.8mol) dropwise, and control the temperature at 20-25°C. After the dropwise addition, stir the reaction at room temperature for 5h, and monitor the raw material (enyl ester) ≤ 0.1% in the liquid phase. Let stand, separate layers, extract the aqueous phase with 2700ml of dichloromethane again, combine the organic phases, and wash with 2700ml of 3% sodium bisulfite solution, 2700ml of 10% sodium carbonate solution, and 2700ml of saturated saline once, Dry over anhydrous sodium sulfate and concentrate to dryness to obtain 445.0 g of crude eplerenone with a molar yield of 95% and an HPLC purity of 98.3%. (See...
Embodiment 2
[0045] Embodiment 2: the preparation of eplerenone crude product
[0046]Add 20g of 9,11-enyl ester, 40.75g of trichloroacetamide, 8.0g of dipotassium hydrogen phosphate trihydrate, and 120ml of dichloromethane into a 500ml three-necked flask in sequence, start stirring, and cool down to 0-5°C under nitrogen protection. 108 ml of 30% hydrogen peroxide solution (0.75 mol) was added dropwise at a temperature of 0-5° C., and the reaction was carried out at a temperature of 20-25° C. for 4-6 hours after the addition was completed. The liquid phase shows that the raw material (enyl ester) ≤ 2.0%, let stand, separate layers, extract the water phase with 120ml of dichloromethane again, combine the organic phase, wash once with 120ml of 3% sodium bisulfite solution, and wash once with 10% sodium carbonate Wash once with 120 ml of the solution, once with 120 ml of saturated saline, dry over anhydrous sodium sulfate, and concentrate to dryness to obtain 18.2 g of crude eplerenone, Y=94....
Embodiment 3
[0047] Embodiment 3: the preparation of eplerenone high-quality goods
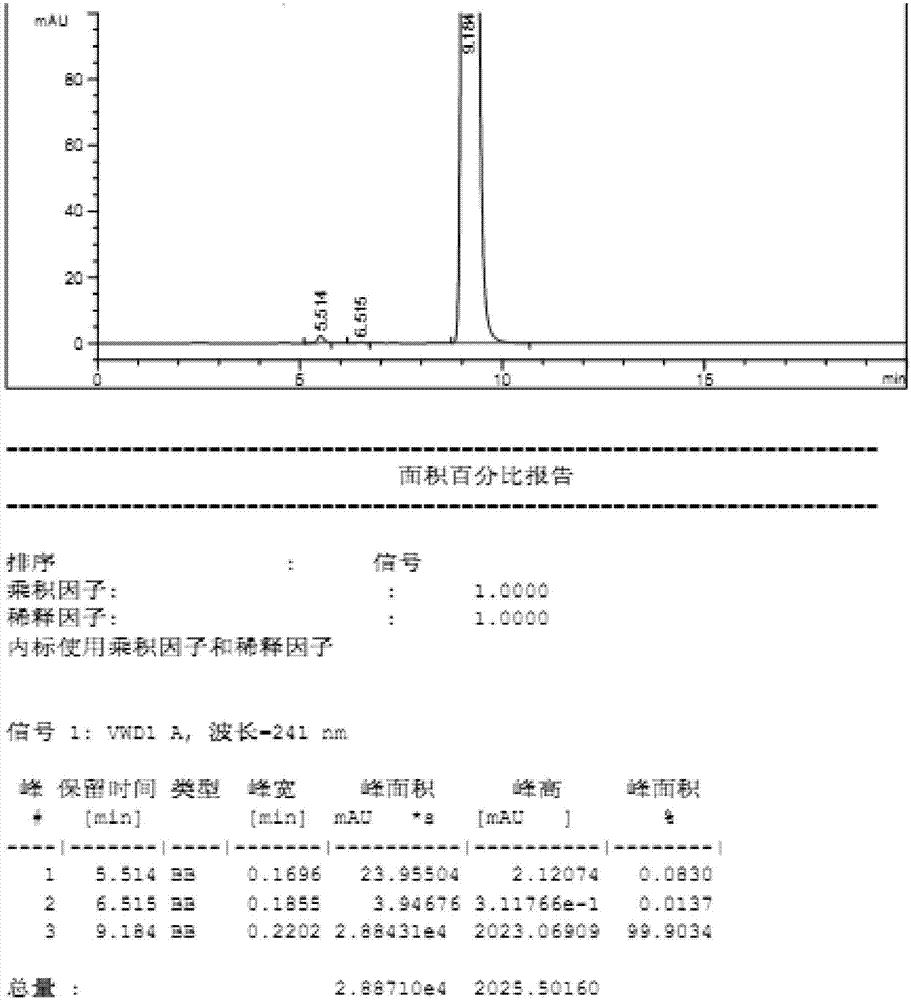
[0048] The crude product obtained in Example 1 was transferred to a 5L glass reaction bottle, 4228ml of 2-butanone was added, the temperature was raised to reflux to dissolve the liquid, the heating was stopped, and the reaction was naturally lowered to room temperature and stirred for 4-8h for crystallization. After filtering, the filter cake was washed with an appropriate amount of 2-butanone, and dried to obtain 400 g of eplerenone fine product, with a refined yield of 90% and a liquid phase purity of 99.9%. (See figure 2 )
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com