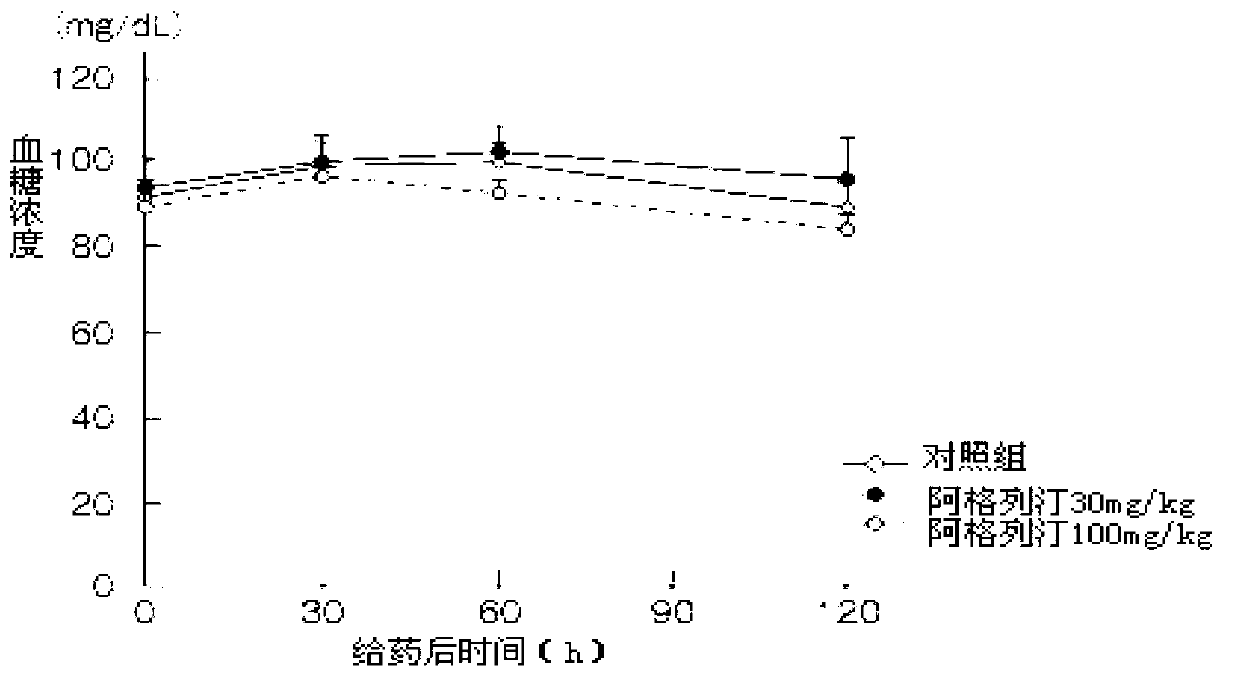
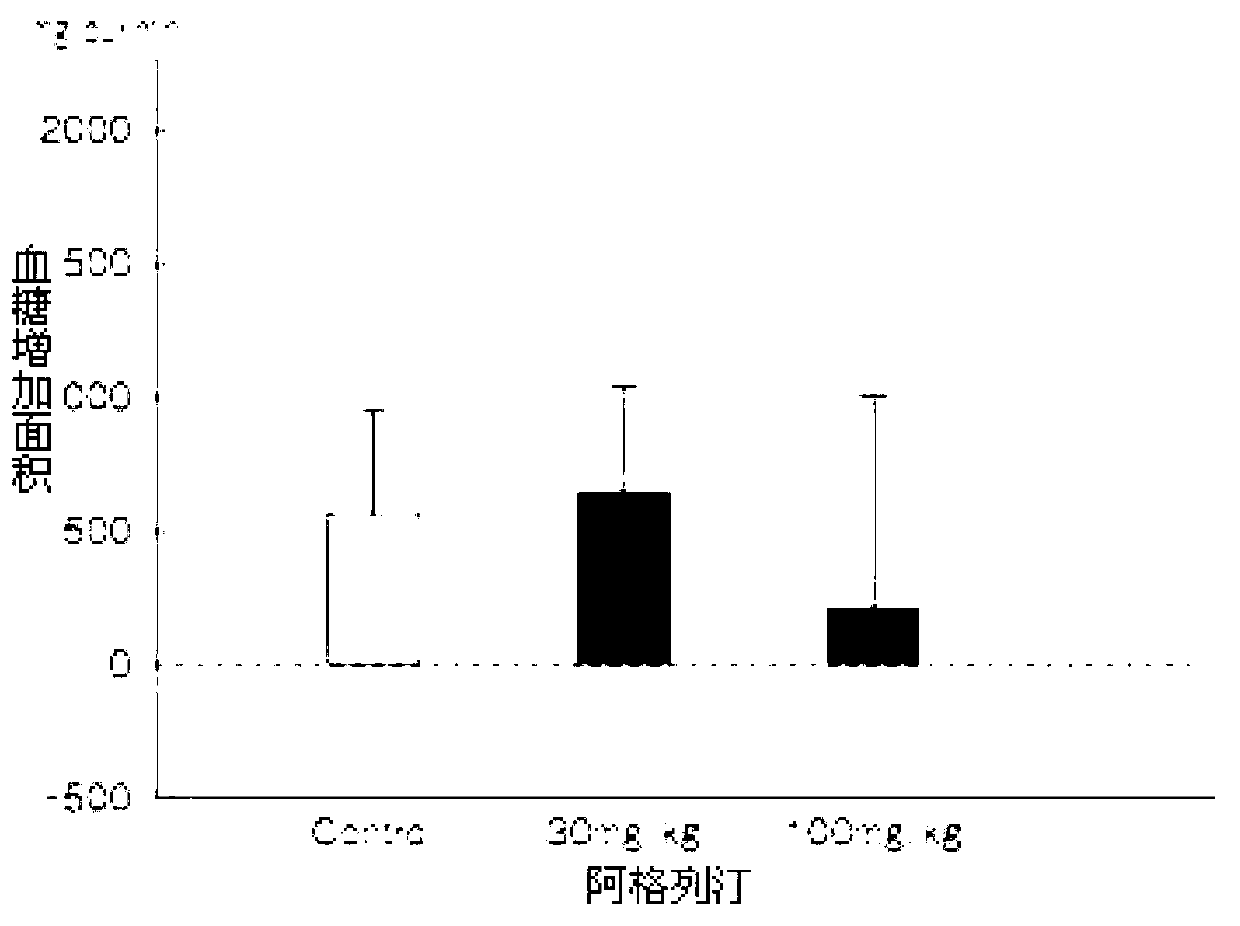
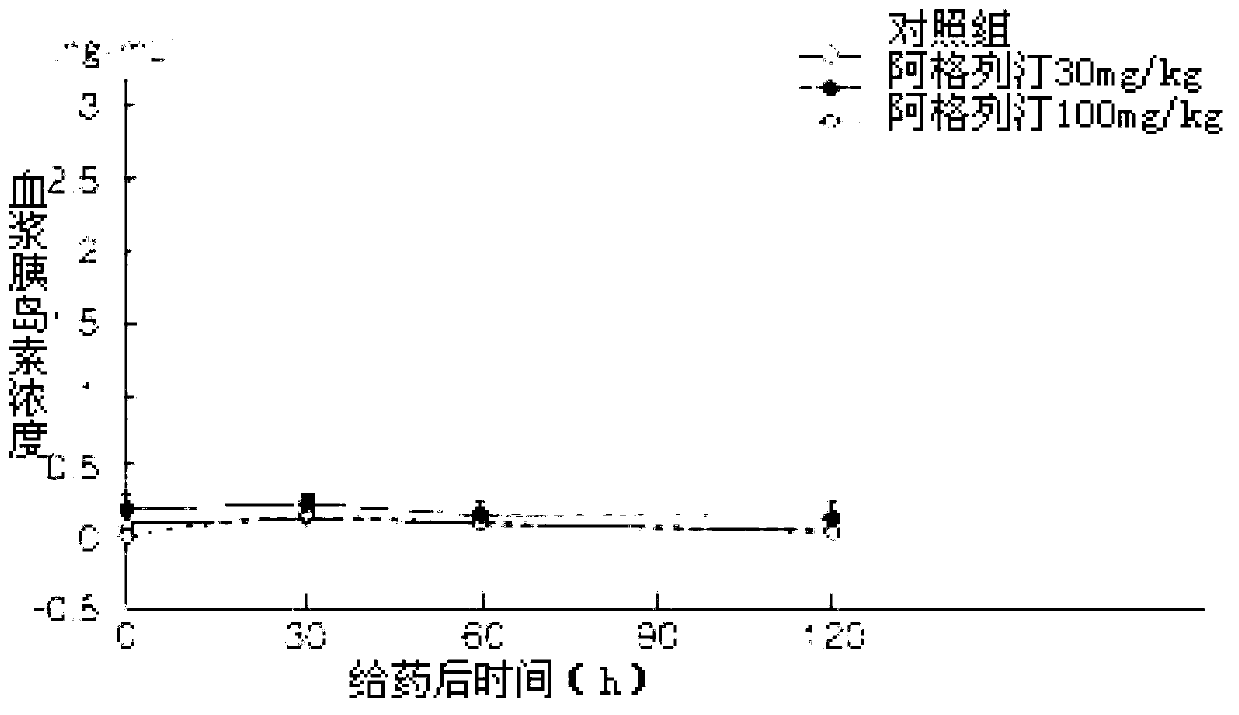
Preparation method of alogliptin benzoate
A technology of benzoic acid and intermediates, which is applied in the field of preparation of alogliptin benzoate, can solve the problems of high price, high cost of preparation raw materials, no weight gain and low blood sugar, and achieves low manufacturing cost and few by-products. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0030] Its specific preparation method comprises the following steps:
[0031] 1) Using nitrogen protection, put 3.5kg of starting material 6-chlorouracil (SM001) into a 100L reactor, add 44L of DMF and DMSO mixed solvent with a volume ratio of 6:1, stir and cool to about -5°C; Add 1.05kg of 60% sodium hydride in batches, keep the temperature of the reaction solution below 0°C, stir for 1 hour after the addition, add 1.85kg of lithium bromide in batches, stir for 1 hour after the addition, dissolve 4.37kg of 2-cyanobenzyl bromide (SM002) Dissolve in 18L of DMF and DMSO in a mixed solvent with a volume ratio of 6:1, then drop it into the above reaction solution, keep the temperature of the reaction solution at -5~0°C, after the addition, keep stirring for 1 hour at the temperature, and heat to room temperature , stirred for 24 hours, TLC detected the reaction (petroleum ether: ethyl acetate = 1:1), the reaction ended when the raw material SM002 disappeared, slowly added about 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com