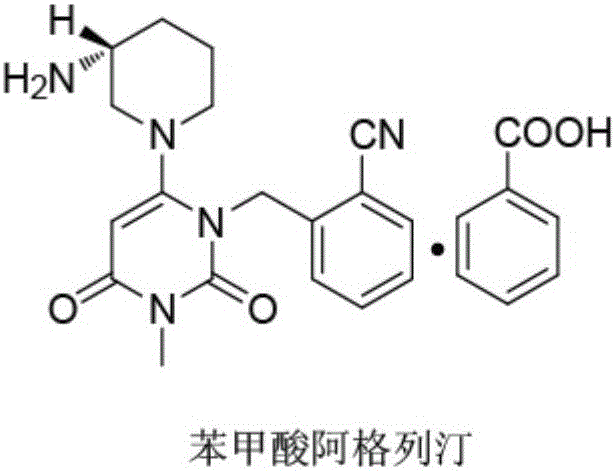
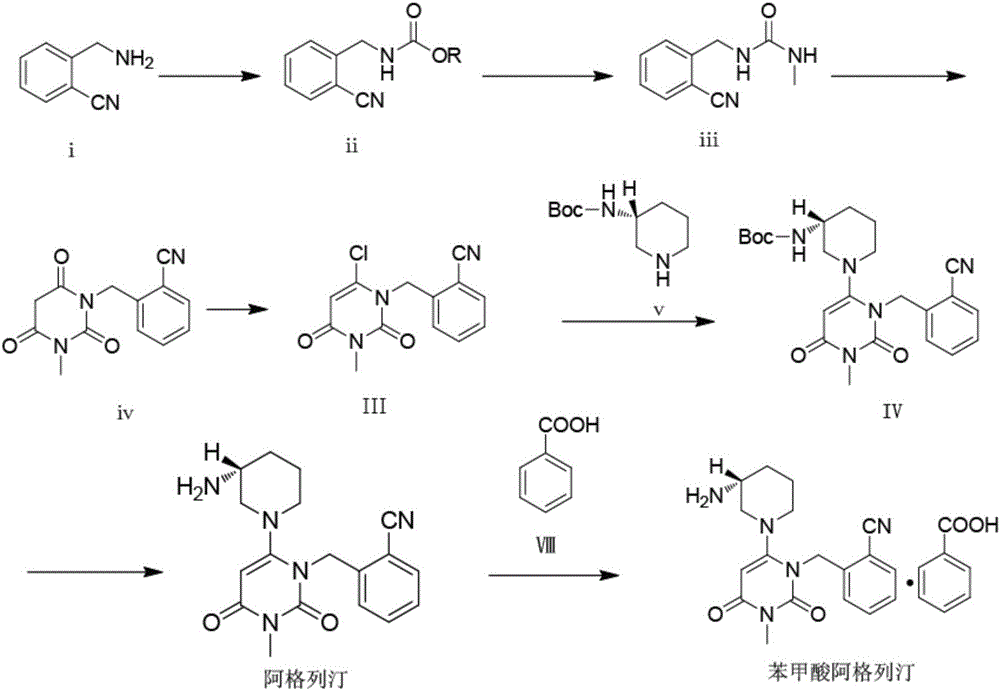
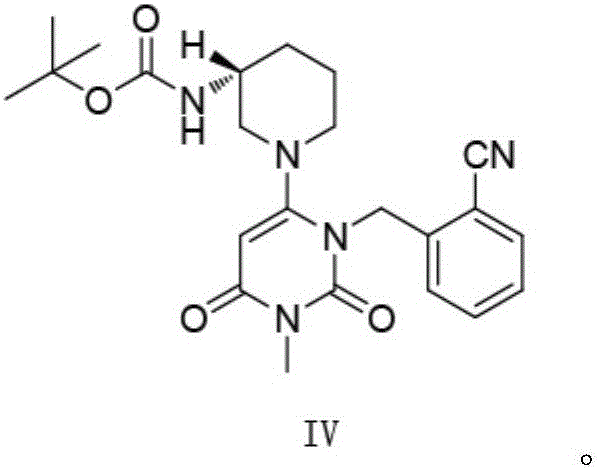
Alogliptin benzoate preparation method
A technology of benzoic acid and formula, applied in the field of preparation of alogliptin benzoate, can solve the problems of difficult feeding, poor washing effect, low product purity, etc., to avoid corrosion of equipment, save production time, and improve reaction rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1: add 0.55 times of mass fraction 10% methanol solution and 3 times of water
[0091] Add 50mL (52g) of the intermediate IV reaction solution into a 250mL three-necked flask, turn on the mechanical stirring, heat up to 65°C, add 28.6g of 10% methanol solution first, no solid matter precipitates, cool down to 25°C, and a large amount of Solid, the solid is loose, does not form agglomerates, does not adhere to the wall, does not stick to the stirring paddle, slowly add 156g of water, stir and wash for 1 hour, filter, and blow dry at 60°C to constant weight to obtain 10.20g of intermediate IV, a pink solid, loss on drying 0.21%, using the HPLC area normalization method to measure the purity of 98.85%, the yield of 81.14%.
Embodiment 2
[0092] Embodiment 2: add 0.55 times of mass fraction 15% methanol solution and 3 times of water
[0093] Add 50mL (52g) of the intermediate IV reaction solution into a 250mL three-neck flask, turn on the mechanical stirring, heat up to 65°C, add 28.6g of 15% methanol solution first, no solids are precipitated, cool down to 25°C, and a large amount of solids are precipitated. The solid is loose, does not form agglomerates, does not adhere to the wall, does not stick to the stirring paddle, slowly add 156g of water, stir and wash for 1 hour, filter, and blow dry at 60°C to constant weight to obtain 10.30g of intermediate IV, a pink solid, with a loss on drying of 0.19% , using the HPLC area normalization method to measure the purity of 98.90%, the yield of 81.94%.
Embodiment 3
[0094] Embodiment 3: adding 0.55 times of mass fraction is 20% methanol solution and 3 times of water
[0095] Add 50mL (52g) of the intermediate IV reaction solution into a 250mL three-necked flask, turn on the mechanical stirring, heat up to 65°C, add 28.6g of 20% methanol solution first, no solids are precipitated, cool down to 25°C, and a large amount of solids are precipitated. The solid is loose, does not form agglomerates, does not adhere to the wall, does not stick to the stirring paddle, slowly add 156g of water, stir and wash for 1 hour, filter, and blow dry at 60°C to constant weight to obtain 10.32g of intermediate IV, a pink solid, with a loss on drying of 0.18% , the purity was 98.83% and the yield was 82.10% as measured by the HPLC area normalization method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com