Industrial production method of Alogliptin benzoate raw material medicine
A technology for benzoic acid and raw materials, applied in the field of industrial production of alogliptin benzoate raw materials, can solve problems such as increased procedures, high production costs, unfavorable production, etc., to reduce the types of solvents used, reduce production costs, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of alogliptin benzoate crude product
[0021]
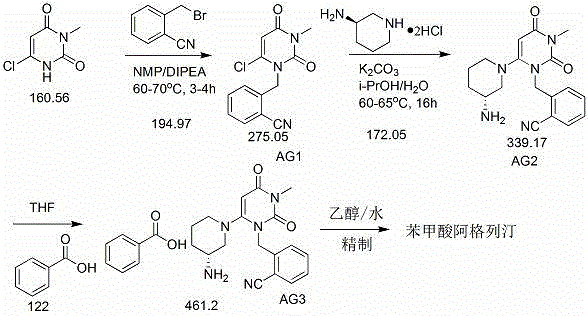
[0022] 1. Preparation of intermediate AG1
[0023] Add 41.2kg of N-methylpyrrolidone, 10.0kg of 6-chloro-3-methyluracil and 12.07kg of diisopropylethylamine into a 100L enamel reaction kettle, and then add 13.43kg of 2-cyanobenzyl bromide into the reaction solution, the temperature was raised to 60~70°C, and the reaction was stirred for 4h. TLC monitoring showed that the raw material point of 6-chloro-3-methyluracil basically disappeared (developer PE:EA=2:1, GF254). Cool the reaction solution to 20-30°C, then slowly add 50.0kg of purified water into the reaction solution, keeping the temperature below 35°C during the process, and continue stirring for 30 minutes after the addition. Cool down to 5~10°C, keep stirring for 1.5h. Centrifuge, wash with 50.0kg of purified water in batches, then add the obtained solid into the reaction kettle, add 39.3kg of isopropanol, stir for 1.5h, centri...
Embodiment 2
[0029] During the implementation of the invention, a comparative study was carried out with the disclosed related technology, and the key process parameters of the present invention were screened and investigated, and the results are shown in Table 1:
[0030]
[0031] It can be seen from the above results that, compared with the disclosed technology, the technology of the present invention is more environmentally friendly, has high yield, higher product purity, and low solvent residue, which is also conducive to the removal of impurity X.
Embodiment 3
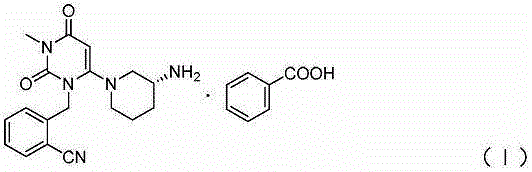
[0033] Add 5.0 kg of alogliptin benzoate crude product, 57 liters of absolute ethanol and 3 liters of water (ethanol / water=95 / 5) into the reaction kettle, heat and reflux until the solution is clear, press filter to a clean area, and cool down to 50°C Add 10.0g of seed crystals, keep warm and crystallize at 40-50°C for 2h, pass cooling circulating water to cool down at 20-30°C and continue to keep warm for 1h, then lower the temperature to 5-10°C and continue to keep warm for 1h, centrifuge, and blow dry at 60°C for 5h. Obtain white alogliptin benzoate finished product 4.52kg, yield 90.4%. The purity is 99.96%, the single impurity is 0.036%, the residual ethanol is 0.07%, and the impurity X is not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com