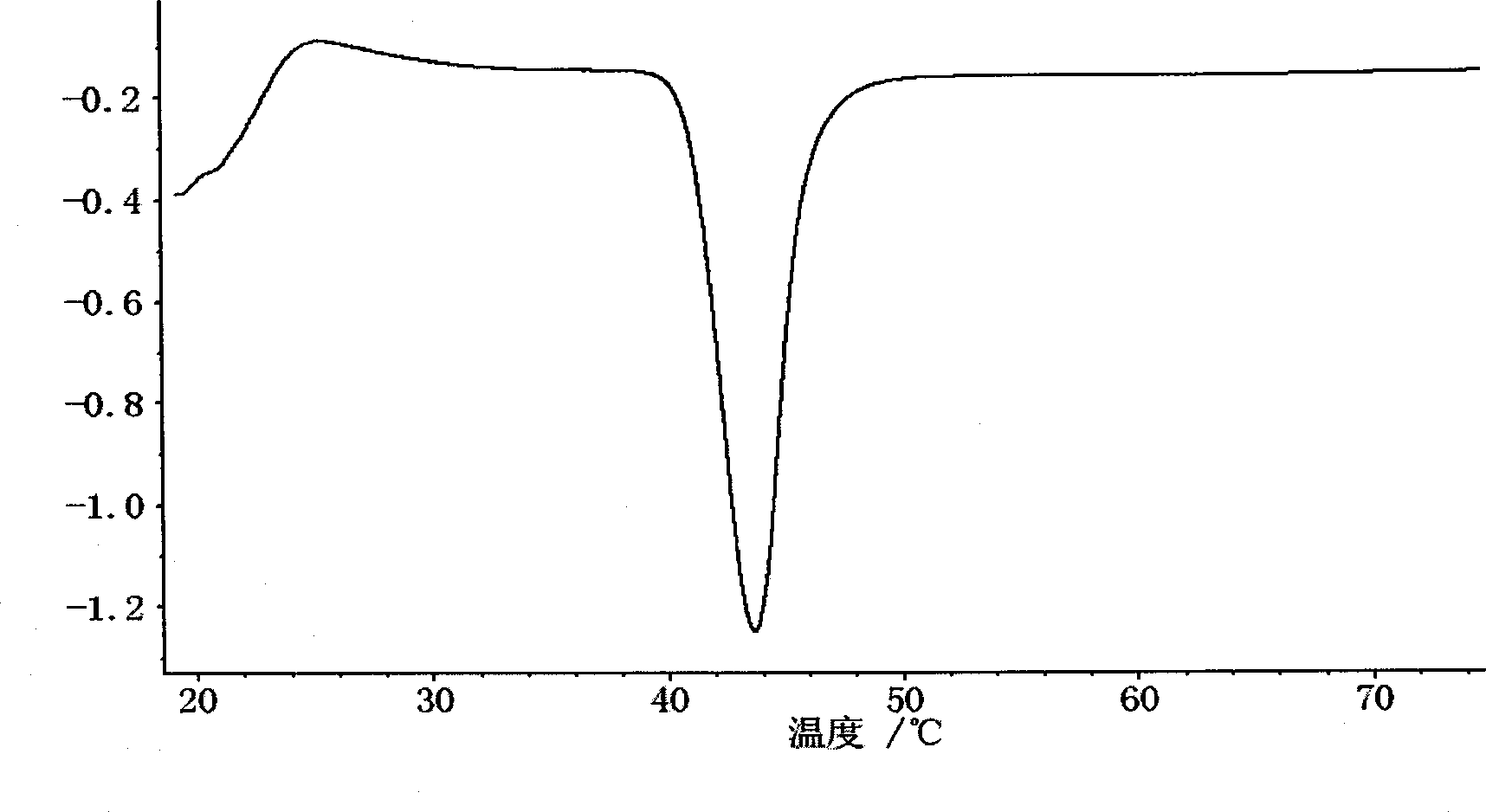
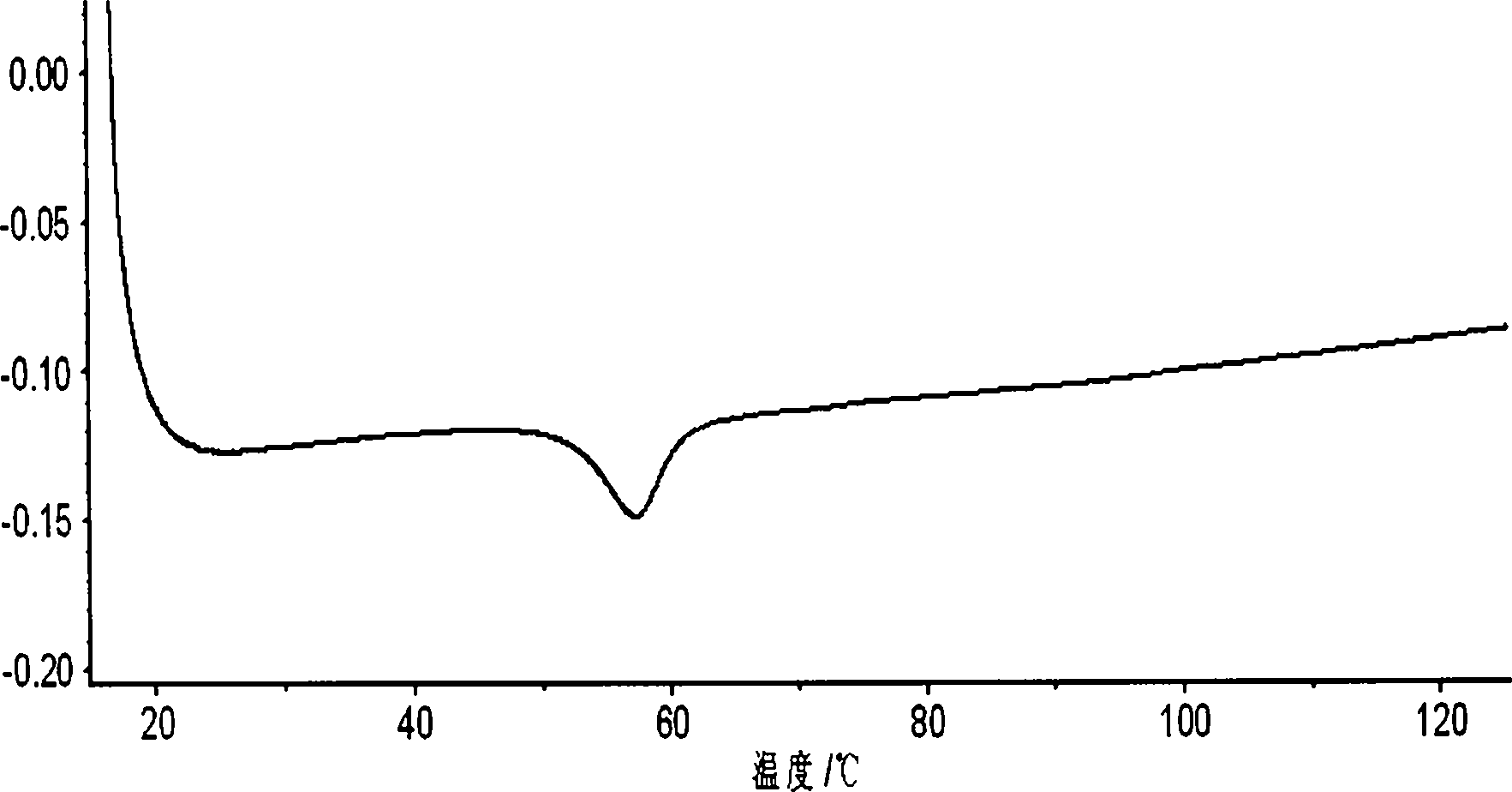
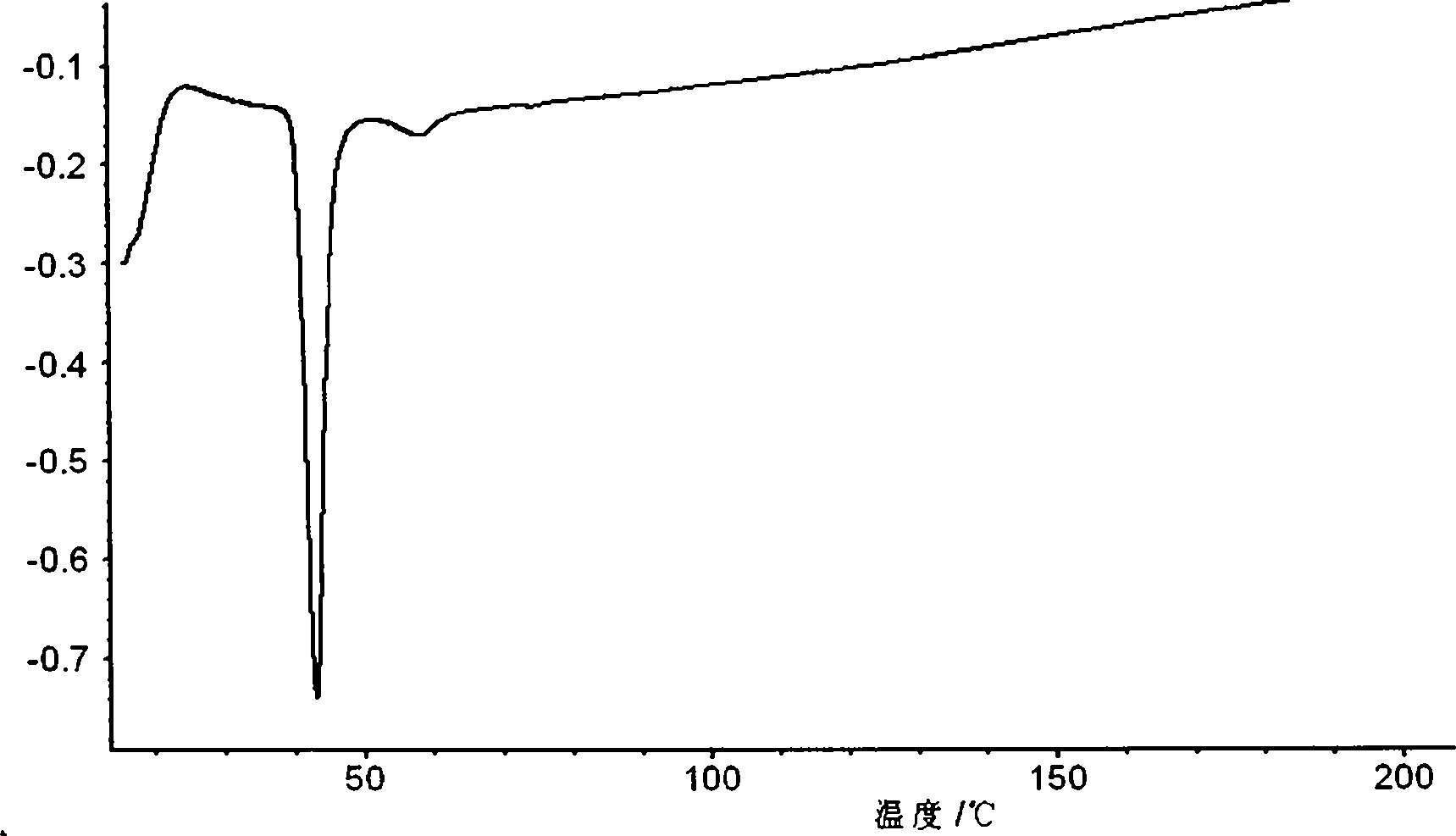
Orlistat oral preparation and preparation method thereof
An orlistat and tablet technology, applied in the field of pharmaceutical preparations, can solve the problems of unimproved dissolution rate of tablets, sticking and punching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Solid Dispersion Preparation
[0034] Orlistat 120g
[0035] Acrylic resin 60g
[0036] Absolute ethanol 600g
[0037] Dissolve the prescribed amount of orlistat and acrylic resin in absolute ethanol, and on the supercritical fluid equipment, pass the supercritical fluid CO2 to quickly depressurize through a nozzle with an aperture of 10 microns, and the decompression time is 10 -5 Second, a solid dispersion of orlistat acrylic resin was prepared by supercritical fluid rapid expansion method, and the D90 of the dispersion was less than 10 μm.
[0038] 2. Preparation of Orlistat Tablets
[0039]
[0040] Mix the solid dispersion with microcrystalline cellulose and crospovidone evenly, add an appropriate amount of pure water, granulate, dry, and pass the dry granules through a 20-mesh sieve, then mix evenly with the prescribed amount of magnesium stearate, and tablet it. have to.
Embodiment 2
[0042] 1. Solid Dispersion Preparation
[0043] Orlistat 120g
[0044]Acrylic resin 360g
[0045] Absolute ethanol 2000g
[0046] Dissolve the prescribed amount of orlistat and acrylic resin in absolute ethanol, and on the supercritical fluid equipment, pass the supercritical fluid CO2 to quickly depressurize through a nozzle with an aperture of 10 microns, and the decompression time is 10 -5 Second, a solid dispersion of orlistat acrylic resin was prepared by supercritical fluid rapid expansion method, and the D90 of the dispersion was less than 10 μm.
[0047] 2. Preparation of Orlistat Tablets
[0048]
[0049] Mix the solid dispersion with microcrystalline cellulose and crospovidone evenly, add an appropriate amount of pure water, granulate, dry, and pass the dry granules through a 20-mesh sieve, then mix evenly with the prescribed amount of magnesium stearate, and tablet it. have to.
Embodiment 3
[0051] 1. Solid Dispersion Preparation
[0052] Orlistat 120g
[0053] Acrylic resin 180g
[0054] Absolute ethanol 1000g
[0055] Dissolve the prescribed amount of orlistat and acrylic resin in absolute ethanol, and on the supercritical fluid equipment, pass the supercritical fluid CO2 to quickly depressurize through a nozzle with an aperture of 10 microns, and the decompression time is 10 -5 Second, a solid dispersion of orlistat acrylic resin was prepared by supercritical fluid rapid expansion method, and the D90 of the dispersion was less than 10 μm.
[0056] 2. Preparation of Orlistat Tablets
[0057]
[0058] Mix the solid dispersion with microcrystalline cellulose and crospovidone evenly, add an appropriate amount of pure water, granulate, dry, and pass the dry granules through a 20-mesh sieve, then mix evenly with the prescribed amount of magnesium stearate, and tablet it. have to.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com