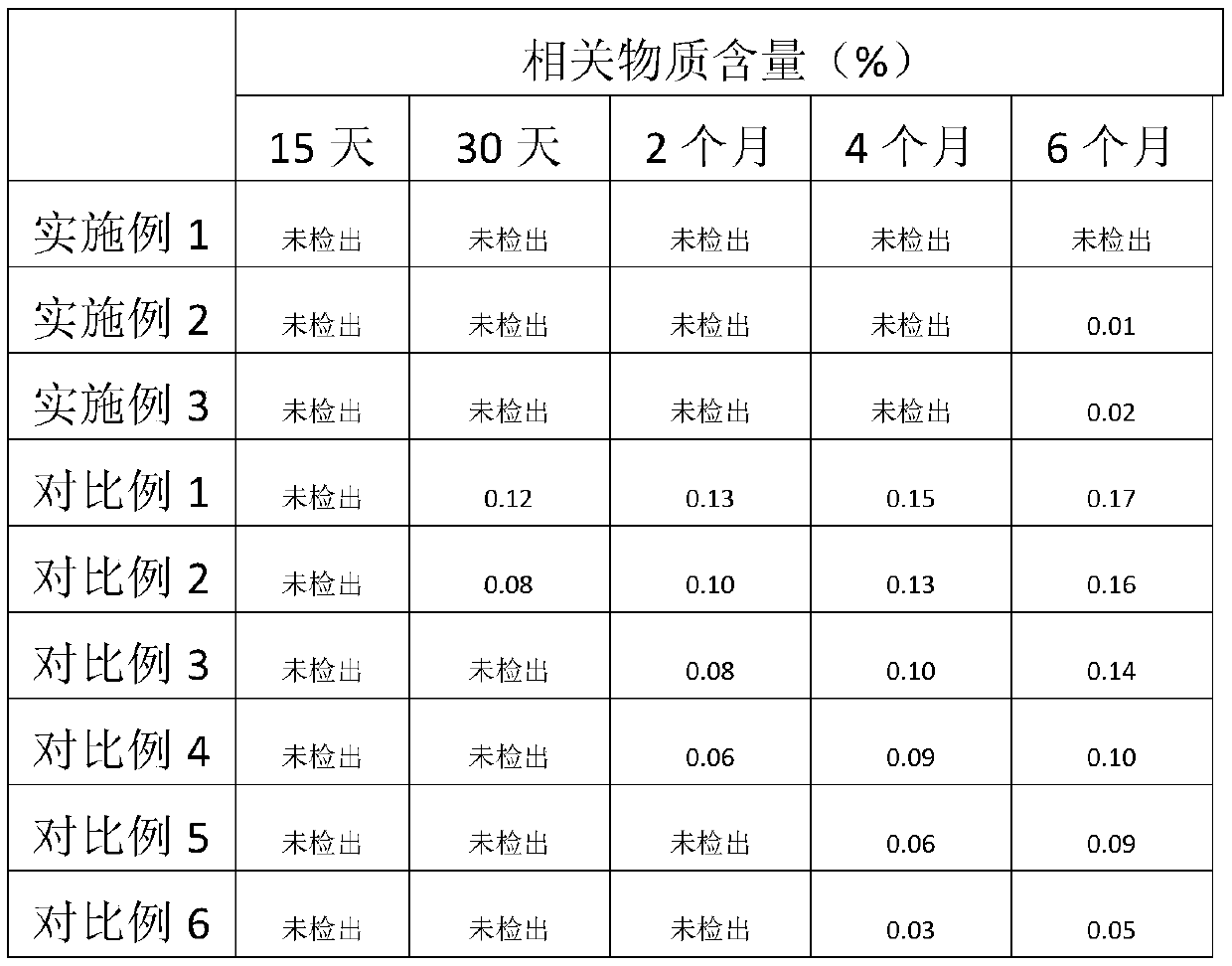
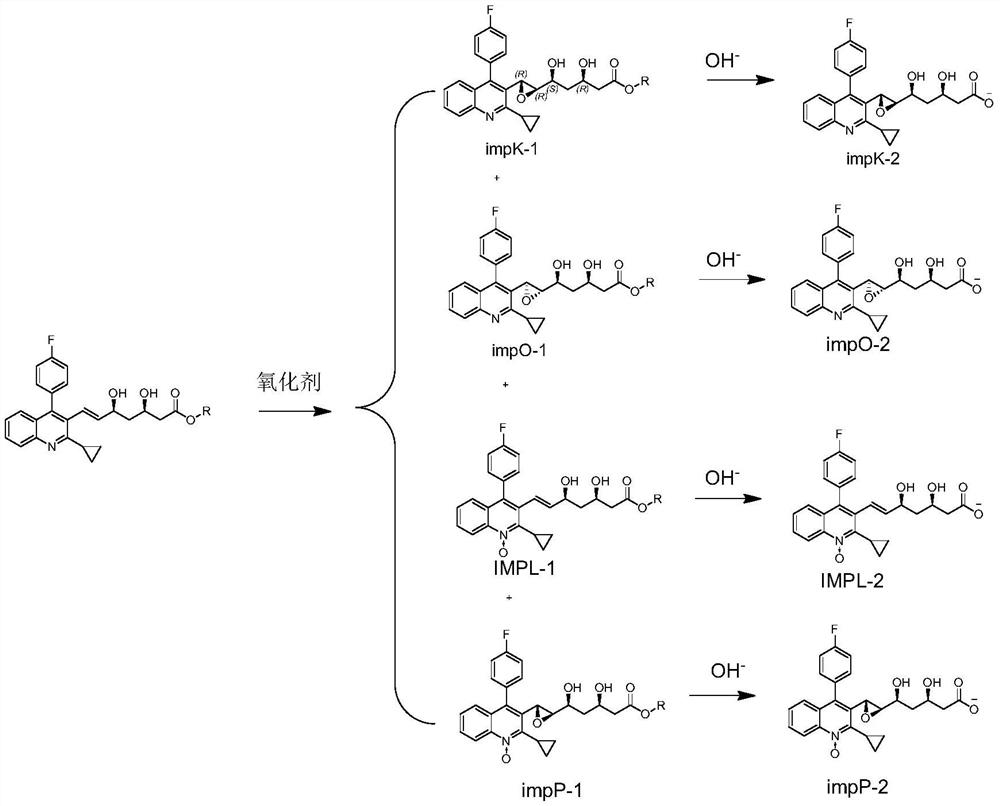
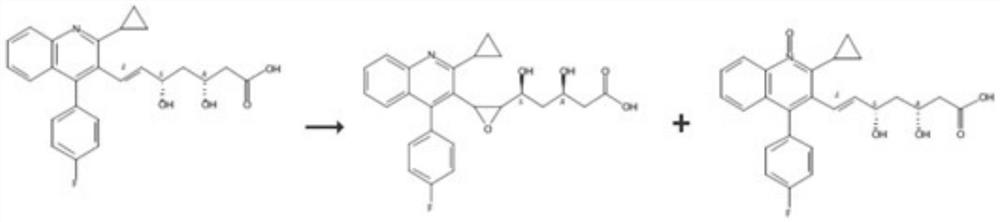
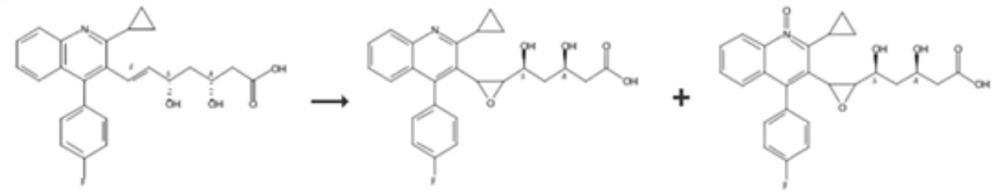
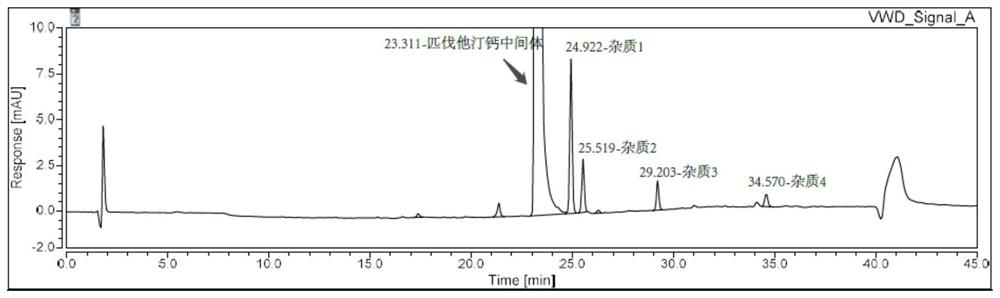
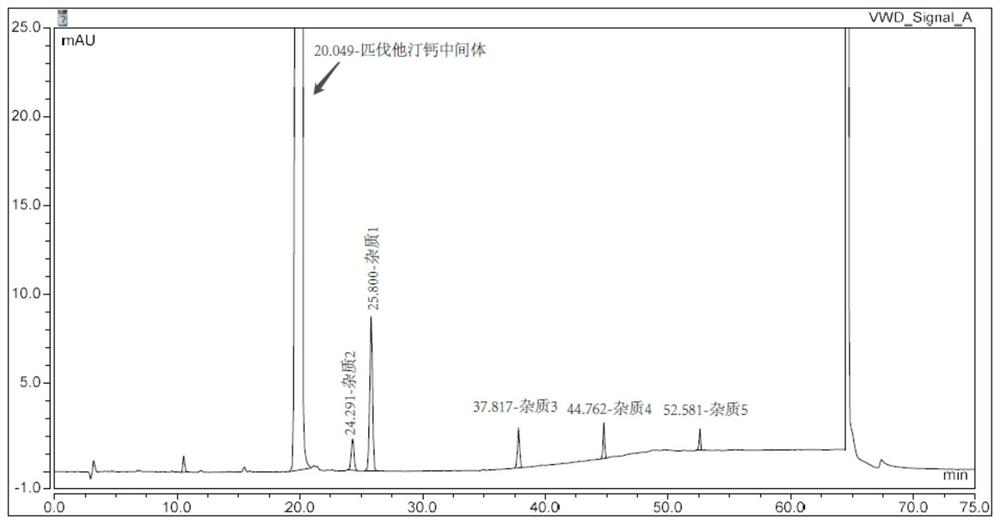
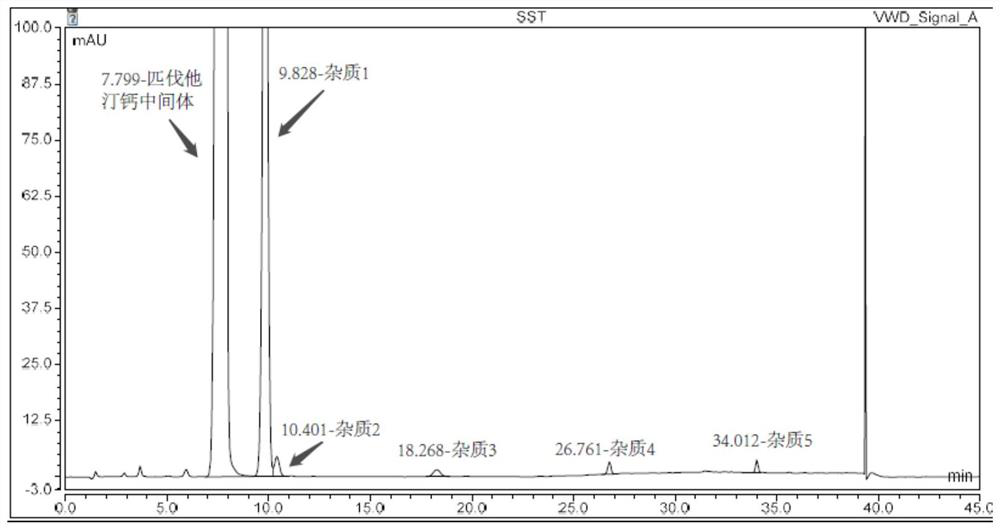
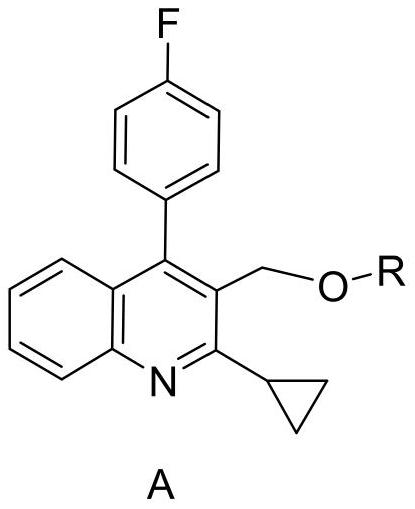
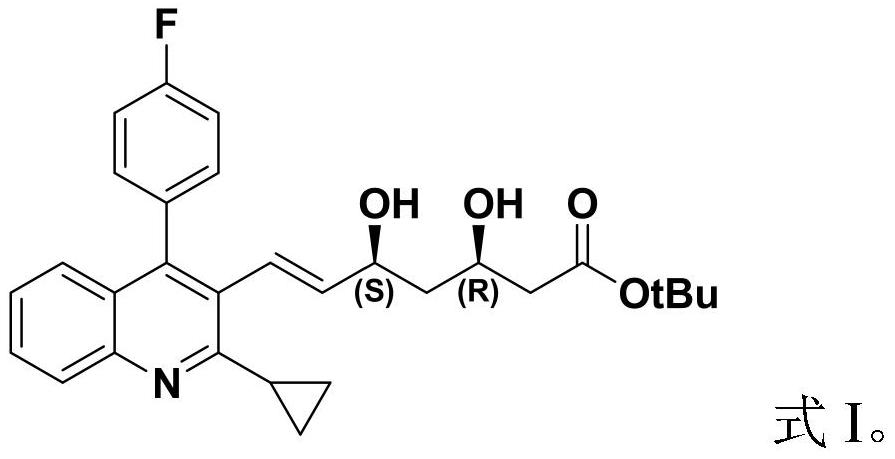
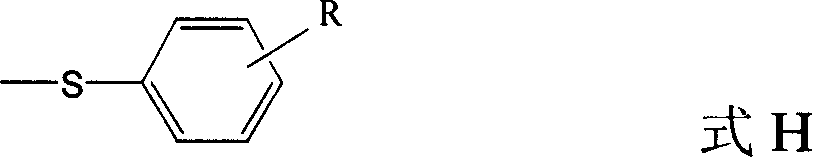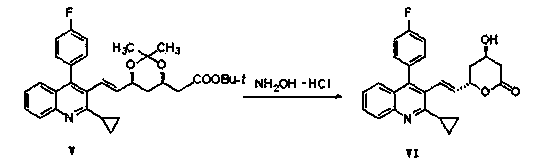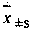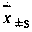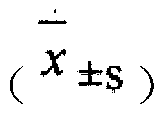Patents
Literature
72 results about "Pitavastatine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quinolines compounds and their intermediates, preparation method and application
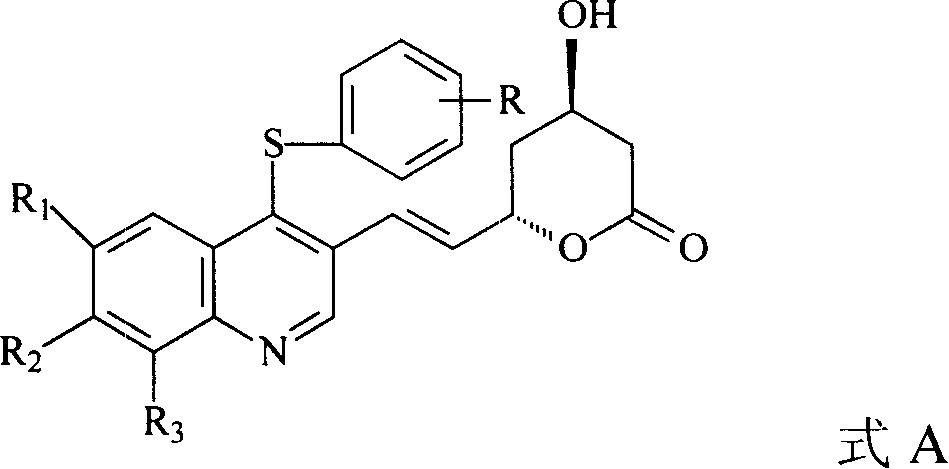
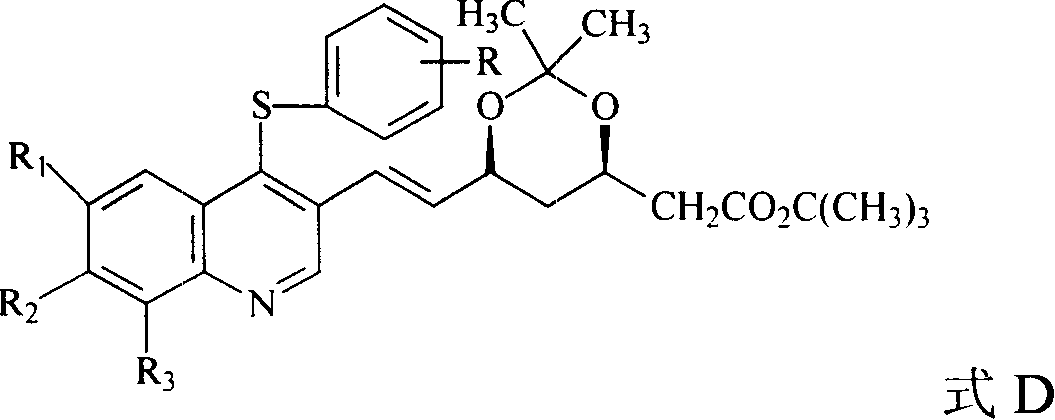
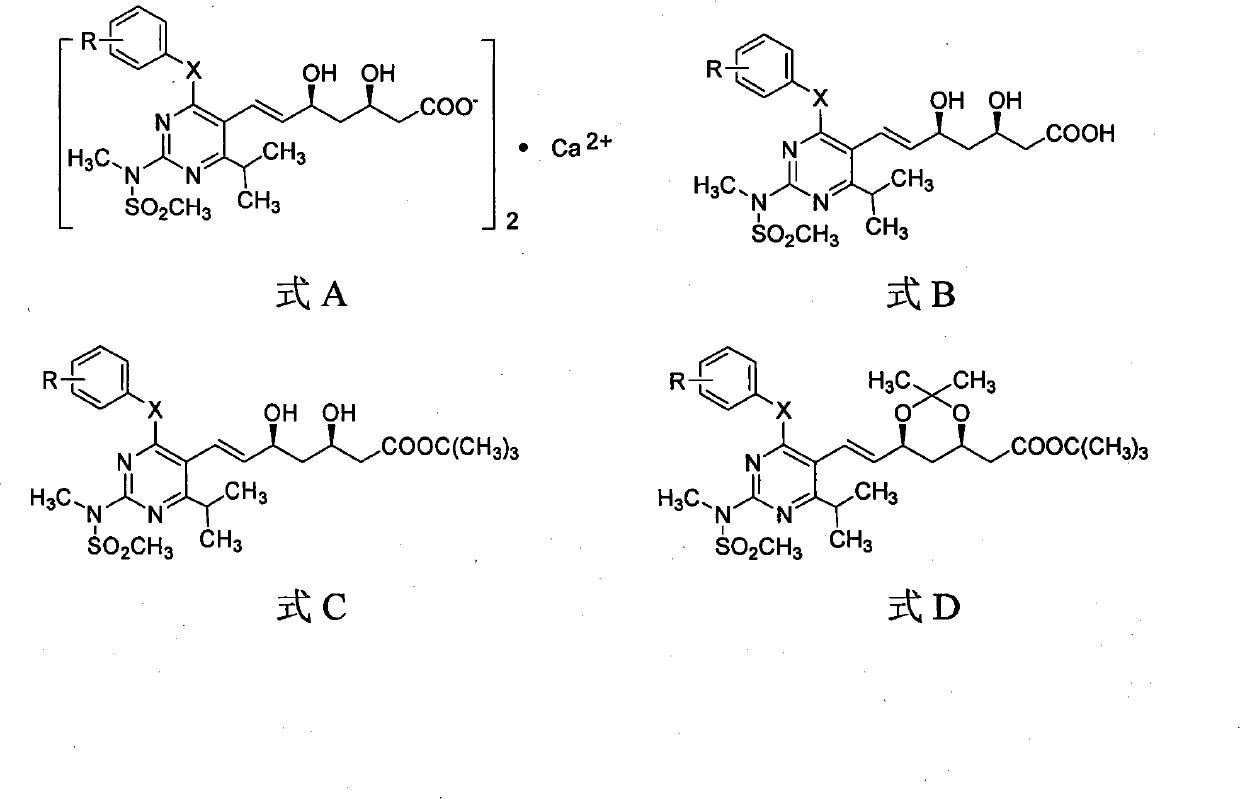
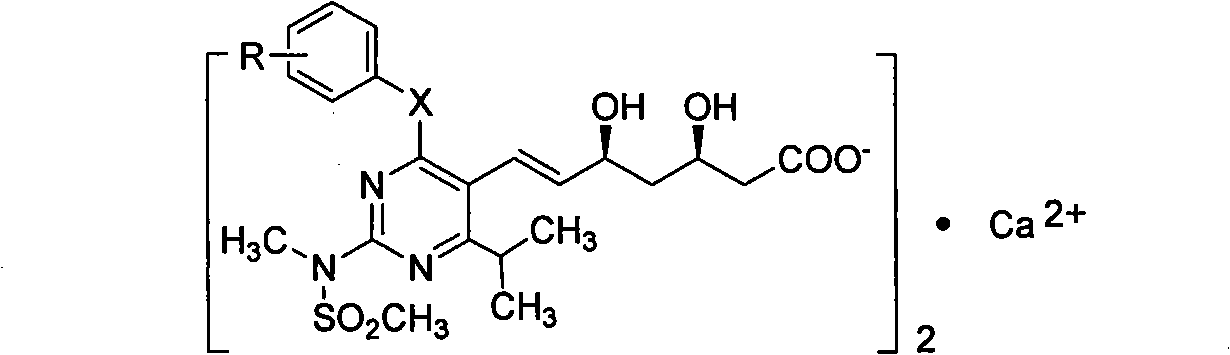
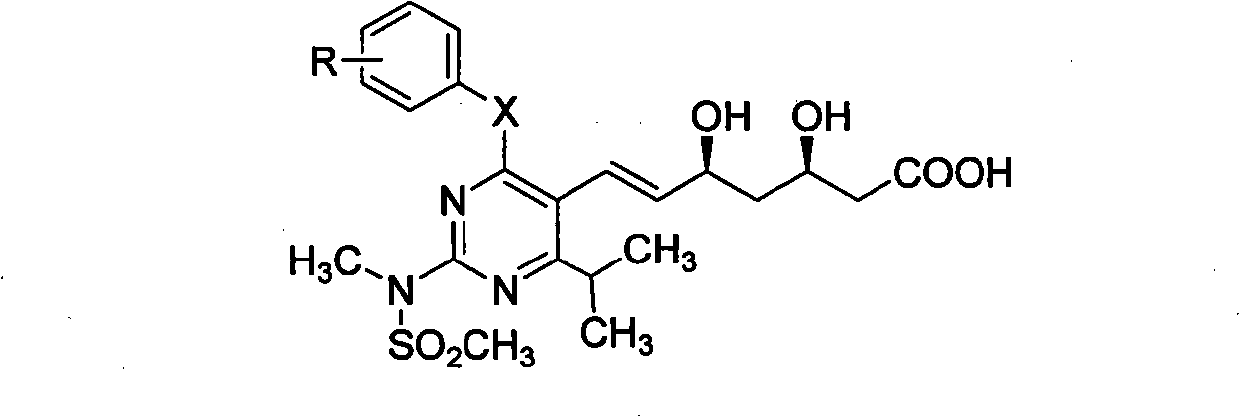
The invention discloses a quinolinic compound showed in the formula A and pharmacy acceptable solvate, optical isomers or polymorphic substance and a reaction intermediate compound showed in the formula D; wherein, R1, R2 and R3 are respective and independent H, halogen or group showed in the formula H; wherein, the R is H, the halogen, alkyl of C1-C4 or alkoxide of C1-C4. The invention further discloses a preparation method thereof and application for preparing medicines for inhibiting HMG CoA reductase and treating hyperlipemia related diseases. Compared with the existing fuvastatin, rosuvastatin and pitavastatin in the prior art, the quinolinic compound of the invention can better inhibiting the activity of the HMG CoA reductase and can be used for treating hyperlipemia related diseases.
Owner:SHANGHAI INST OF PHARMA IND
Novel pitavastatin calcium oral disintegrating tablet composition and preparation method thereof
InactiveCN104367560ASubstance increaseMetabolism disorderPill deliveryDrugs preparationsPharmaceutical Aids
The invention relates to the field of medicinal preparations, and in particular relates to a novel pitavastatin calcium oral disintegrating tablet composition and a preparation method thereof. The composition is mainly formed by combining soluble auxiliary materials and insoluble auxiliary materials in a certain proportion. The pitavastatin calcium composition has the characteristic of being rapidly disintegrated in the oral cavity to release medicines so as to promote effects of absorption and utilization.
Owner:万全万特制药江苏有限公司
Preparation method of pitavastatin calcium
ActiveCN103508946AAchieve halogenationHigh yieldPhosphorus organic compoundsHydroxylamineCholesterol
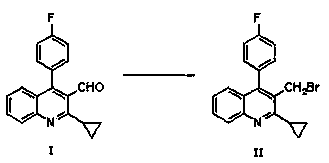
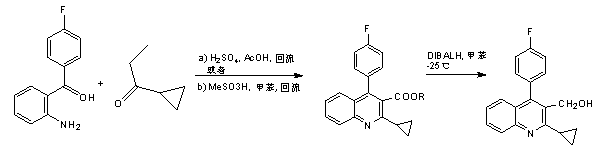
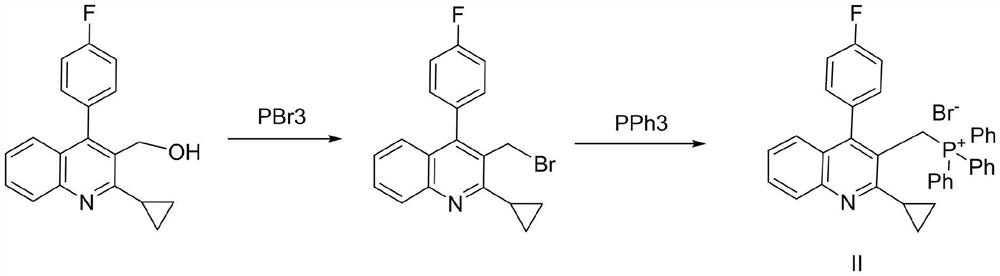
The invention relates to a preparation method of a cholesterol reduction drug, particularly relates to a preparation method of pitavastatin calcium as a crude drug of the cholesterol reduction drug, and aims at the problems that the pitavastatin calcium synthetic technology in the prior art is long in steps and complicated in operation, and uses strongly corrosive reagents which is environmentally unfriendly, causes serious corrosion to equipment, and may not facilitate industrial production. The invention provides the new preparation method of the pitavastatin calcium, the new preparation method is as follows: 3-bromomethyl-2-cyclopropyl-4-(4-fluorophenyl)quinoline is prepared from 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinecarboxaldehyde by a one step reaction, and then reacts with an organophosphorus reagent to obtain pitavastatin calcium intermediate phosphorus ylide, on the basis of improving of the yield to 80%, the reaction steps are reduced, and the reaction difficulty is reduced, and hydroxylamine hydrochloride is selected as a deprotection reagent, so that the new preparation method is mild in reaction conditions, environmentally friendly, high in yield, and beneficial to industrial production.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
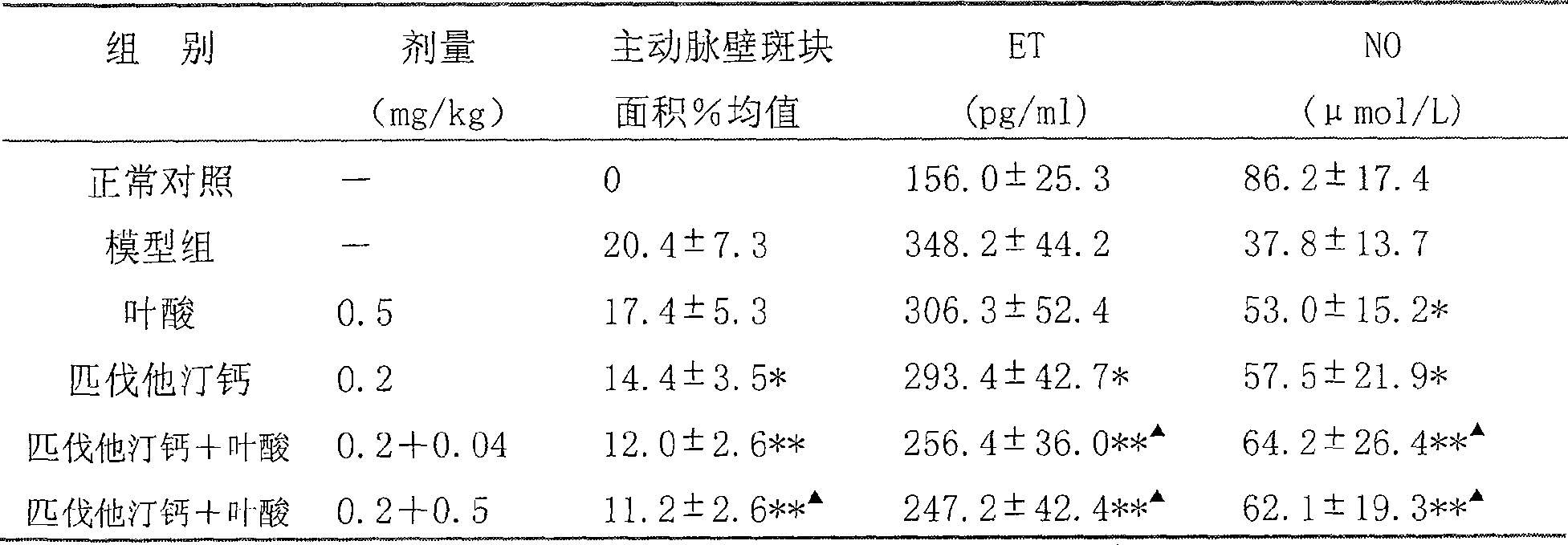
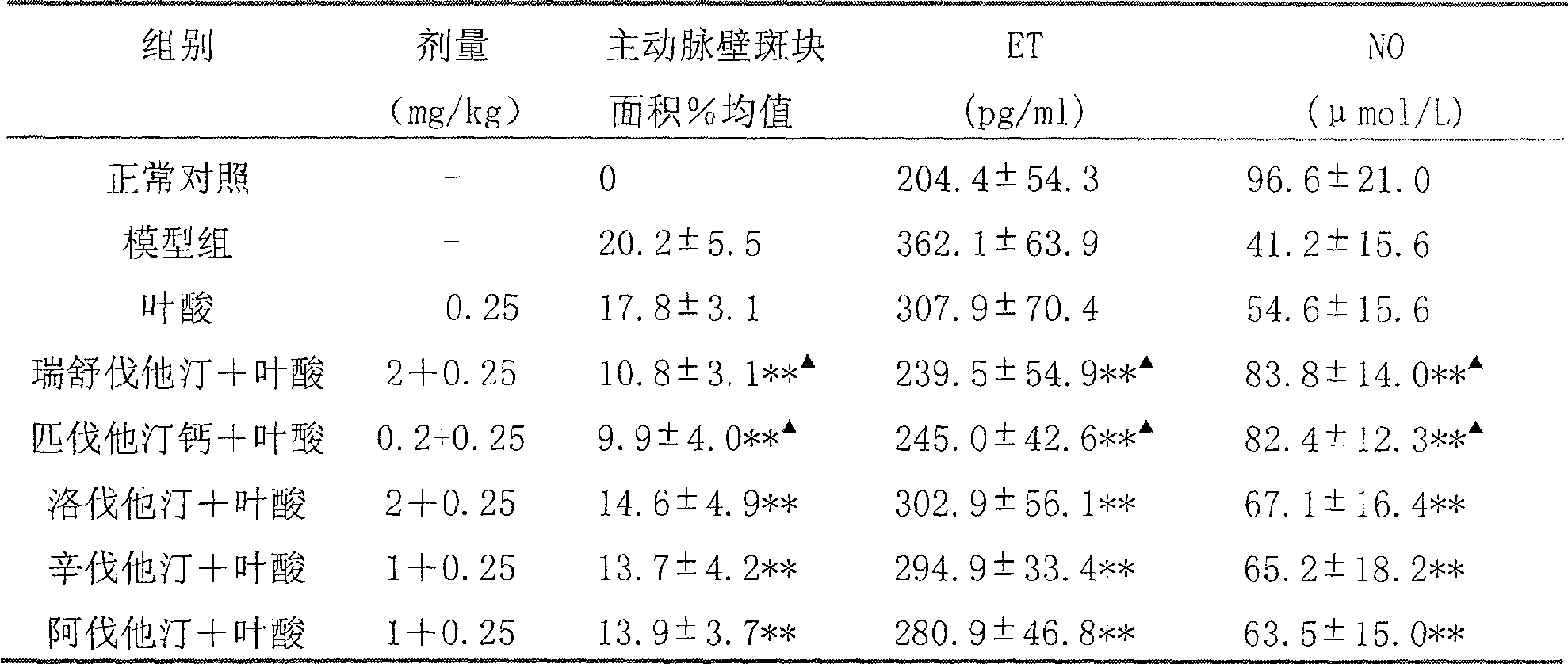
Medicinal composition containing rosuvastatin or pitavastatin and B clan vitamin
ActiveCN1891219AGood curative effectImprove compliancePill deliveryGranular deliveryDiseasePitavastatin
The present invention relates to a medicine composition conlaining resulvatatine or pivatatine compound and vitamins B, in which the resulvatatine or pivatatine content is 1-40 mg and vitamins B content is 0.1-50 mg. Said invention also provides the application of said medicine composition in preparation of medicine for preventing and curing the diseases of atherosclerosis, angiocardiopathy and cerebrovascular disease.
Owner:SHENZHEN AUSA PHARM CO LTD
New method of biologically synthesizing (R)-3-hydroxylglutarate monoester
The invention relates to a new method of biologically synthesizing (R)-3-hydroxylglutarate monoester and more particularly provides a method of preparing the (R)-3-hydroxylglutarate monoester from (S)-4-chloro-3-hydroxybutyrate through halogenohydrin dehalogenase recombinant bacteria and nitrilase recombinant bacteria, or double-enzyme co-expression recombinant bacteria, in a one-pot manner. The (R)-3-hydroxylglutarate monoester is a key intermediate of statin medicines such as fluvastatin, rosuvastatin, pitavastatin and the like. The method is mild in reaction conditions, is free of pollution and is simple in process route.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
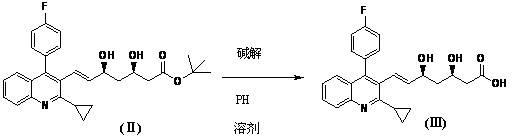
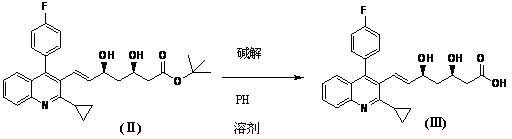
Preparation method of pitavastatin calcium intermediate compound
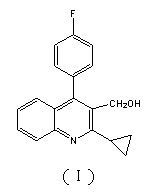
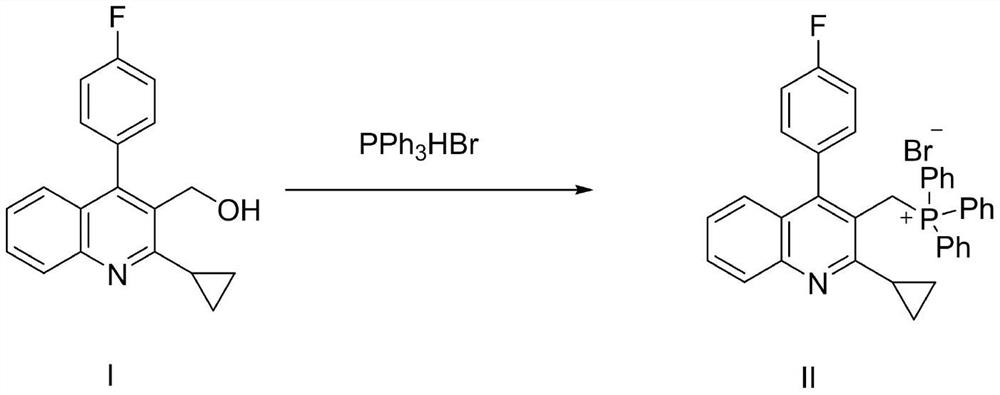
The invention relates to a preparation method of a pitavastatin calcium intermediate compound which has a chemical name of 2-cyclopropyl-3-hydroxymethyl-4-(4-fluorophenyl)-quinoline and is shown in Formula (I). The pitavastatin calcium intermediate compound is prepared by hydrolyzing and reducing an ester compound shown in Formula (II) under specific conditions.
Owner:湖北丰融医药有限公司
Miazine compounds, intermediates of miazine compounds, preparation method of intermediates and miazine compounds as well as application of miazine compound
InactiveCN102079726AInhibits reductase activityOrganic active ingredientsOrganic chemistryHMG-CoA reductaseSecondary hyperlipidemia
The invention discloses miazine compounds as shown in a formula A which is disclosed in the specification and reaction intermediate compounds as shown in formulas B, C and D, wherein X is O or S, R is H,F,C1-C3 alkyl or C1-C3 alkoxy. The invention also discloses preparation methods of the intermediates and miazine compounds and an application of the miazine compounds in preparing medicaments for inhibiting HMG-CoA reductase and / or treating hyperlipidemia diseases. Compared with the pitavastatin, osuvastatin and atorvastatin in the prior art, the 6-isopropyl-2-(N-methyl-N-sulfonyl) amino-4-substituted phenoxy (or thiphenyl) miazine compounds have better or at least comparative activity for inhibiting the HMG-CoA reductase, and can be used for treating the hyperlipidemia diseases.
Owner:SHANGHAI INST OF PHARMA IND
Synthetic method of pitavastatin tert-butyl ester
ActiveCN111875538AHigh stereoselectivityHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystQuinoline
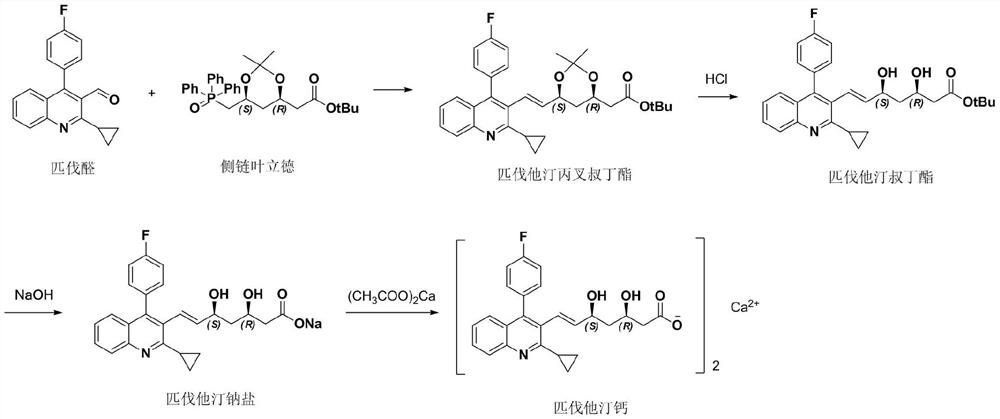
The invention discloses a synthesis method of pitavastatin tert-butyl ester, which comprises the following steps: carrying out a reaction on (4R-Cis)-6-chloromethyl-2,2-dimethyl-1,3-dioxolane-4-tert-butyl acetate with a substance A under the action of a first base catalyst to obtain a substance B; oxidizing the substance B with an oxidizing agent to obtain a substance C; carrying out a reaction with 2-Cyclopropyl-4-(4-fluorophenyl)-quinoline-3-formaldehyde under the action of a second base catalyst to obtain a substance D; and finally, performing acid deprotection to obtain the pitavastatin tert-butyl ester. The method has the advantages of mild and controllable reaction conditions, wherein the reaction conditions of Julia olefination are free of requirement of ultralow-temperature reaction, so that the synthesis method has advantages of convenience and simplicity in operation, good stereoselectivity and high yield, and the synthesized pitavastatin tert-butyl ester is completely free of cis-isomer and high in purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
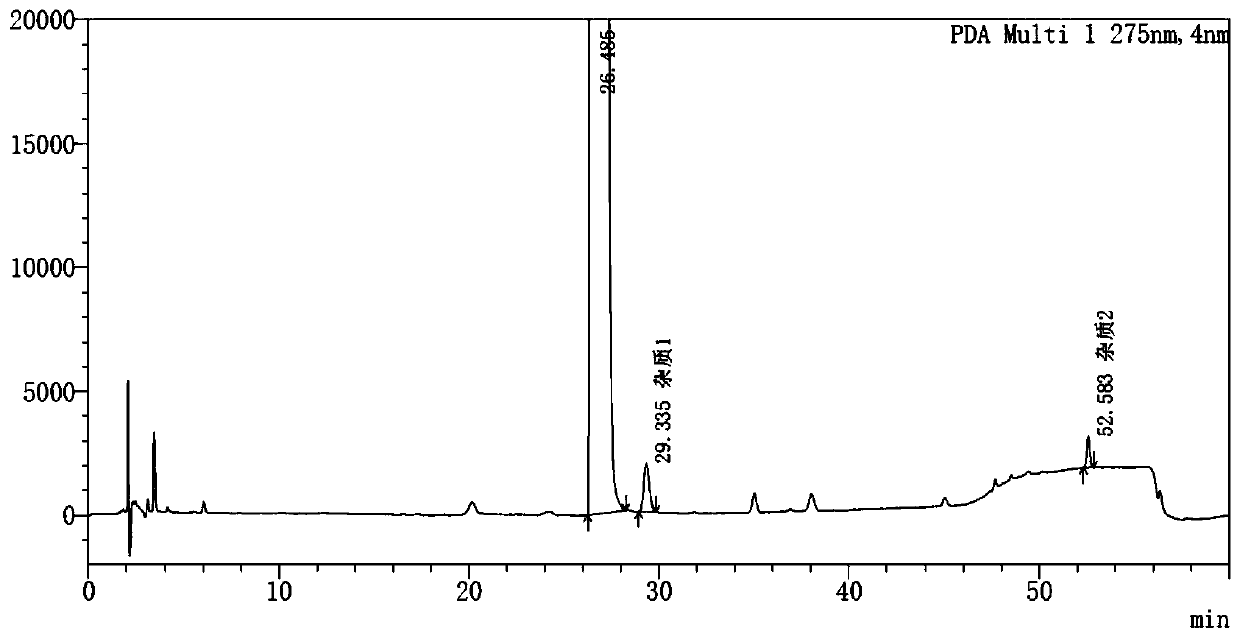
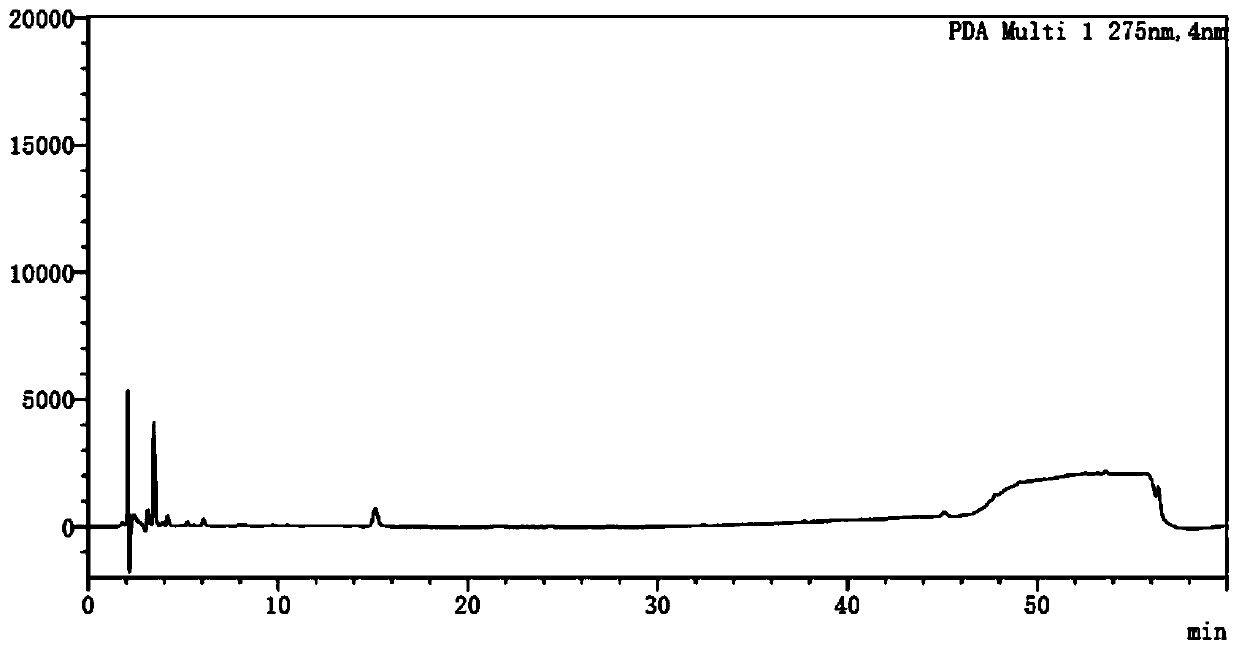
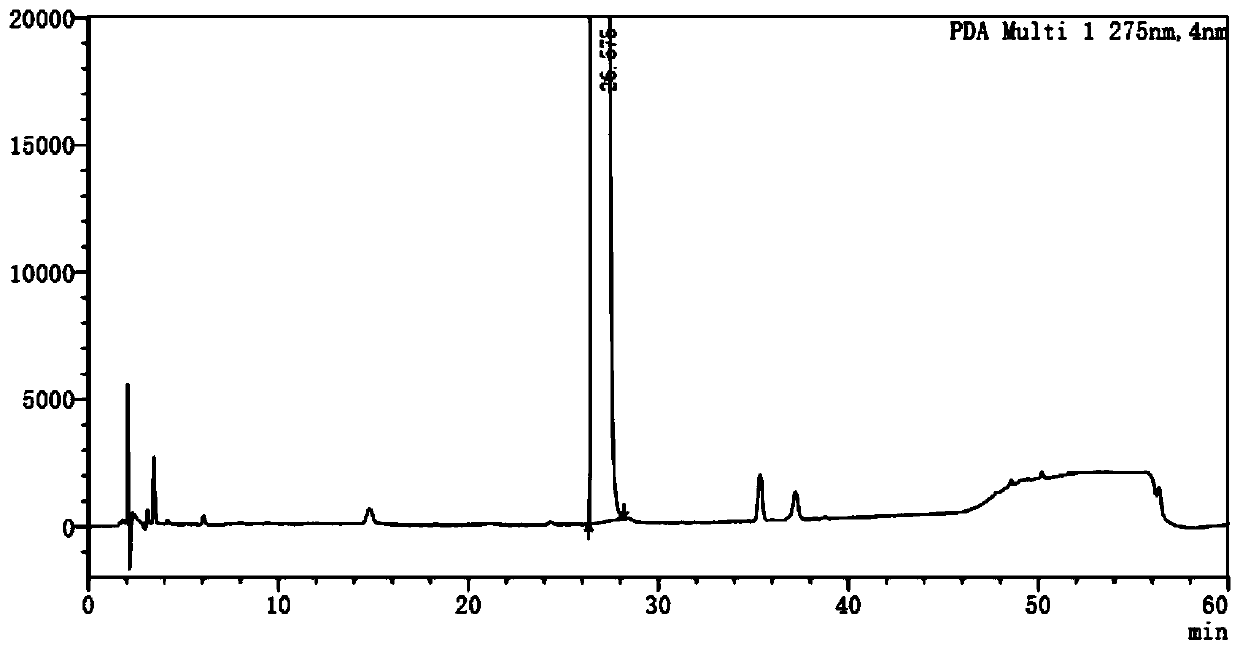
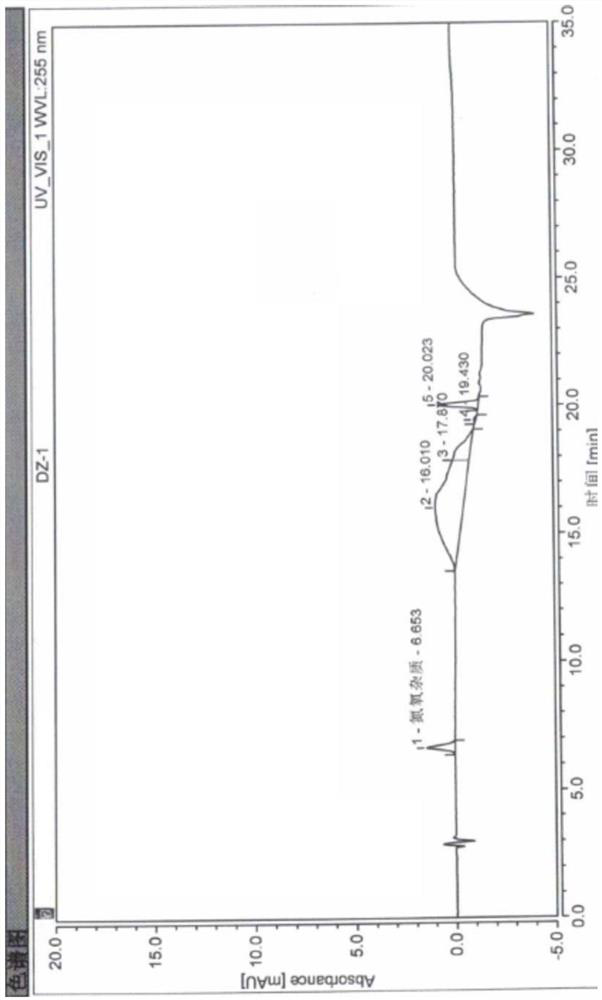
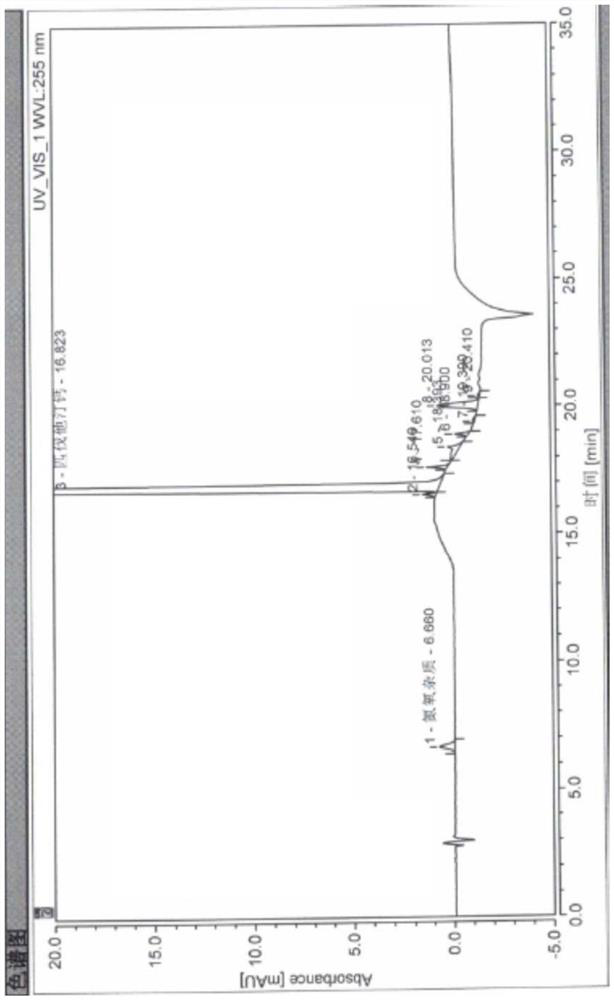
Method for detecting potential mutagenic impurities in pitavastatin calcium tablets
InactiveCN111505150AAvoid interferenceEffective monitoring of drug qualityComponent separationGradient elutionSolvent
The invention relates to the field of medicine quality detection, in particular to a method for detecting potential mutagenic impurities in pitavastatin calcium tablets. The method comprises the following steps: dissolving a to-be-detected sample by adopting a solvent, and detecting by adopting a high performance liquid chromatography under the chromatographic conditions that a mobile phase comprises an organic acid salt buffer solution and acetonitrile; the flow rate is 1.0 mL / min; the column temperature is 25-40 DEG C; a chromatographic column: octadecylsilane chemically bonded silica is used as a filler; the sample injection volume is 50 [mu] L; the detection wavelength ranges from 270 nm to 280 nm; an elution mode is gradient elution; the potential mutagenic impurities are an impurity1 and an impurity 2. According to the method disclosed by the invention, the pitavastatin calcium, the impurity 1 and the impurity 2 can be effectively separated; meanwhile, interference of other related impurities of pitavastatin calcium on detection of the impurity 1 and the impurity 2 is avoided, the content of potential mutagenic impurities 1 and 2 of the pitavastatin calcium tablets can be accurately detected, the quality of the pitavastatin calcium tablets is effectively monitored, and the medication safety is improved.
Owner:SHANDONG QIDU PHARMA
Medicine
InactiveUS20150164809A1Good disintegrationMaintain stabilityBiocideMetabolism disorderCrospovidonesPitavastatin
The present invention provides a pharmaceutical product which includes a solid preparation comprising pitavastatin or a salt thereof, in which production of a lactone form thereof is suppressed.The pharmaceutical product is characterized by including a solid preparation comprising the following ingredients (A) and (B): (A) pitavastatin or a salt thereof; and (B) at least one member selected from the group consisting of carmellose and a salt thereof, crospovidone, and microcrystalline cellulose, and the solid preparation having a water content of 2.9 mass % or less, wherein the solid preparation is stored in a tight package.
Owner:KOWA CO LTD
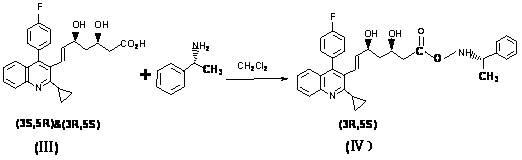
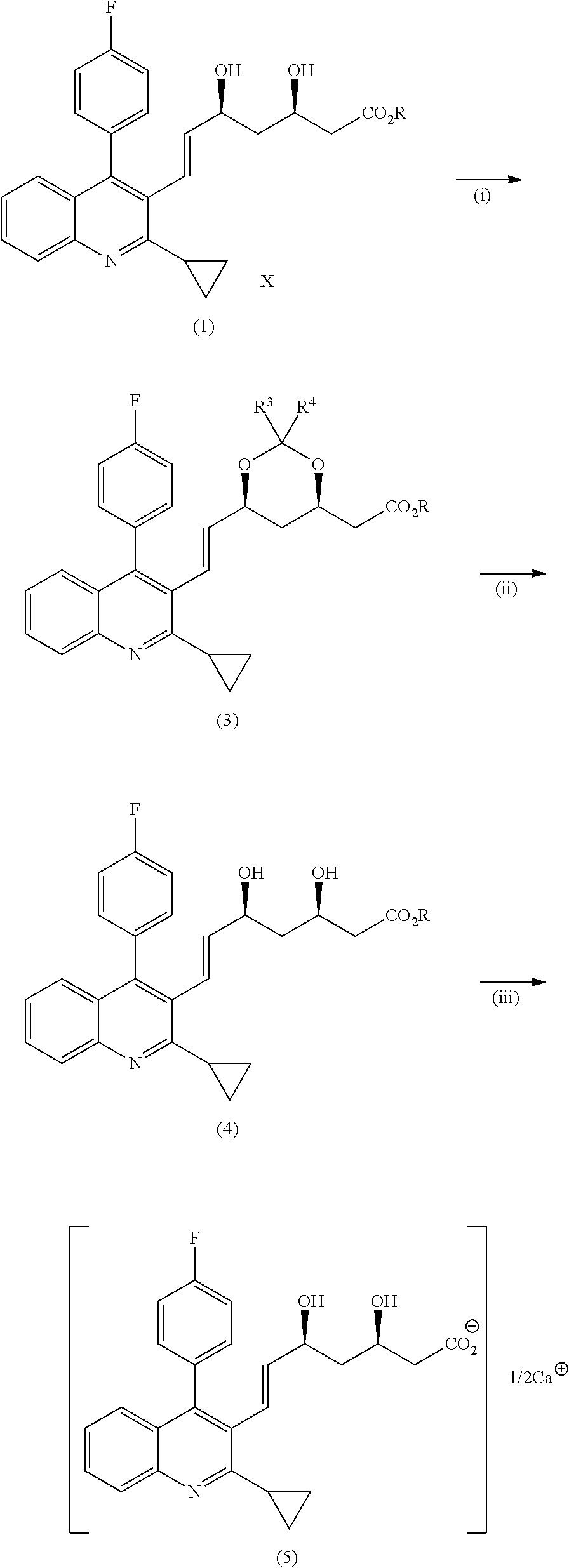
Method for preparing high-optical purity pitavastatin calcium
The invention relates to a method for preparing high-optical purity pitavastatin calcium. The method comprises the following steps of: deprotecting (3R,5S)-dyhydroxyl-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dyhydroxyl-3,5-Oisopropylidene-6-tert-butyl heptenoate (I) serving as an initial raw material by using acid, and removing impurities under a certain pH value; adding excessive D-(+)benzyl methylamine, and crystallizing to extract heptenoic acid amine salt (IV); and adding calcium chloride to obtain the pitavastatin calcium. The method has the advantages that the reaction condition is mild, the purity of the product is high, the optical purity is more than or equal to 99.5 percent, the yield is more than or equal to 50 percent, the problems of high difficulty of separation and purification and low yield in the conventional method for synthesizing the pitavastatin calcium, and the method is suitable for industrialization.
Owner:ZHEJIANG GUOBANG PHARMA
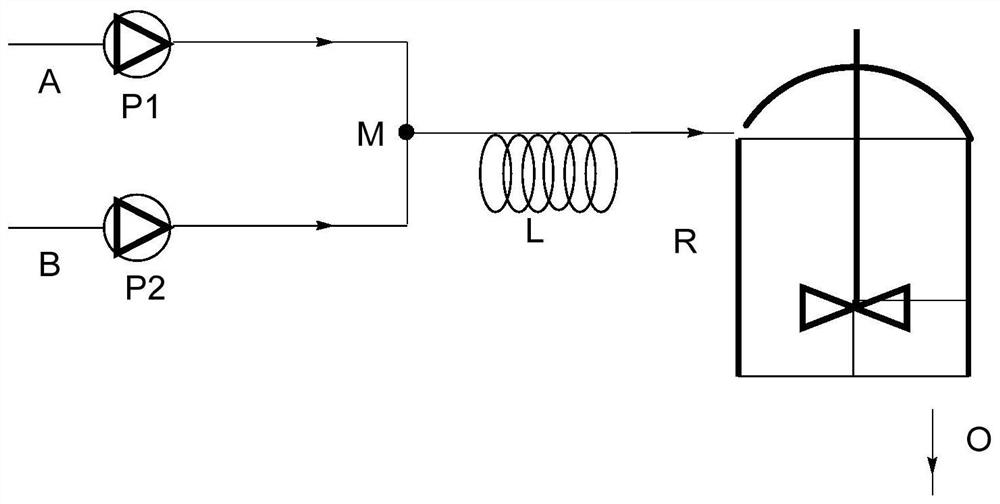
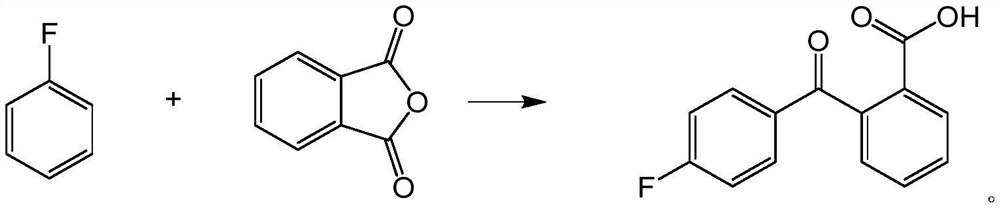
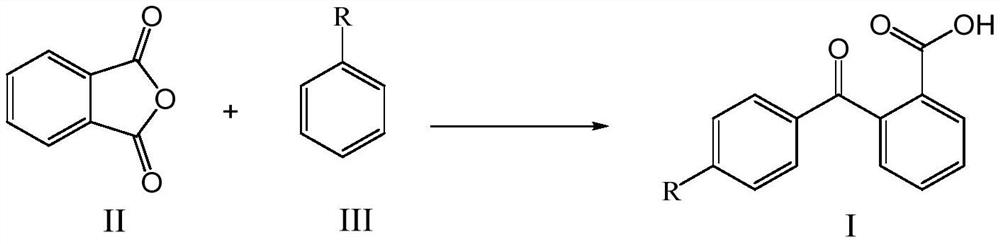
Method for synthesizing pitavastatin calcium intermediate by microchannel reactor
PendingCN113149824AReduced activityPoor yieldPreparation from carboxylic acid anhydridesChemical/physical/physico-chemical microreactorsBenzoic acidPtru catalyst
The invention relates to a method for synthesizing a pitavastatin calcium intermediate in a microchannel reactor. Fluorobenzene and phthalic anhydride are used as raw materials, a target product 2-(4-fluoro-benzoyl)-benzoic acid is synthesized through the microchannel reactor, and in the reaction process, and a carbonate compound is used for replacing aluminum trichloride in the prior art to serve as a catalyst, so the catalyst treatment before industrial production is avoided, meanwhile, a large amount of aluminum-containing wastewater is not generated in the reaction process, the method is green and environment-friendly, the reaction condition is mild, the reaction time is short, the product yield is high and reaches 92% or above, the purity is high and reaches 99% or above, the product post-treatment is simple, the cost is reduced, and the method is suitable for industrial production.
Owner:JIANGSU ALPHA PHARM CO LTD
Alpha-glycosidase inhibitor and hydroxymethyl glutaryl coenzyme A reductase inhibitor composition for treating diabetes and complications
The invention discloses an alpha-glycosidase inhibitor and hydroxymethyl glutaryl coenzyme A reductase inhibitor composition for treating diabetes and complications. The medicinal composition consists of an alpha-glycosidase inhibitor or a pharmaceutically acceptable salt thereof, a hydroxymethyl glutaryl coenzyme A reductase inhibitor or a pharmaceutically acceptable salt thereof and a medicinal carrier or an excipient, wherein the alpha-glycosidase inhibitor is acarbose, voglibose or miglitol; and the hydroxymethyl glutaryl coenzyme A reductase inhibitor is simvastatin, lovastatin, fluvastatin, pravastatin, pitavastatin, rosuvastatin, atorvastatin, cerivastatin, mevastatin or rosuvastatin. The invention also relates to application of the medicinal composition in treatment of diabetes and prevention and treatment of diabetic complications such as diabetic nephropathy, diabetic hypertension, hyperlipidemia and dyslipidemia.
Owner:乔文龙
Pitavastatin calcium lipid solid preparation
InactiveCN102302452AQuality improvementImprove bioavailabilityMetabolism disorderPharmaceutical non-active ingredientsDipalmitoyl PhosphatidylcholineCurative effect
The invention discloses a pitavastatin calcium lipid solid preparation and a preparation method thereof. The preparation method comprises the following steps: preparing pitavastatin calcium lipid with excellent quality from pitavastatin calcium, DPPC (dipalmitoyl phosphatidylcholine), stearamide, octadecylamine and Tween 80 in a specific weight proportion; and preparing the pitavastatin calcium lipid into a solid preparation by a conventional preparation method. Compared with the existing preparation, the preparation disclosed by the invention has the advantages of greatly improved stability and bioavailability, enhanced quality, reduced toxic and side effects, smooth and stable drug release, and remarkable curative effect.
Owner:HAINAN MEIDA PHARMA
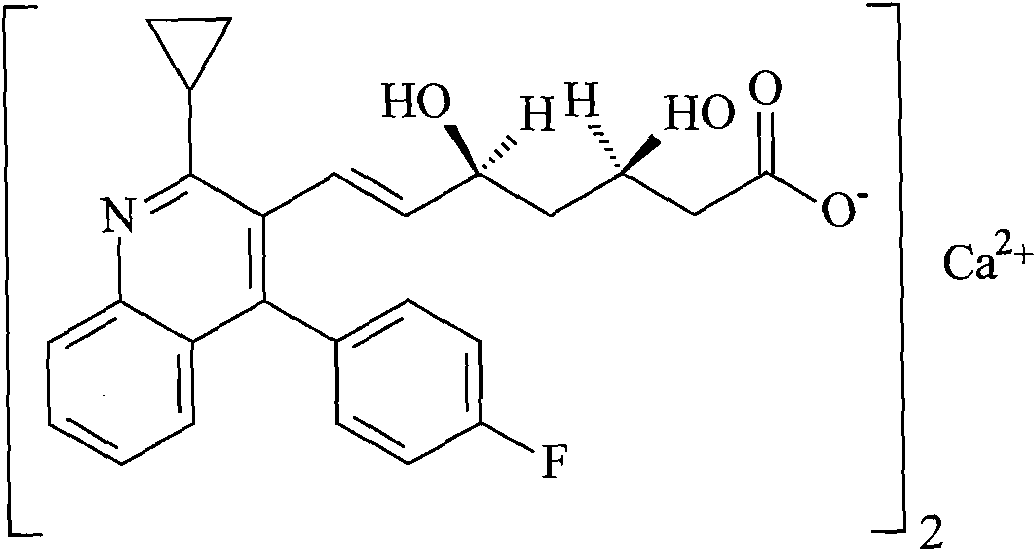
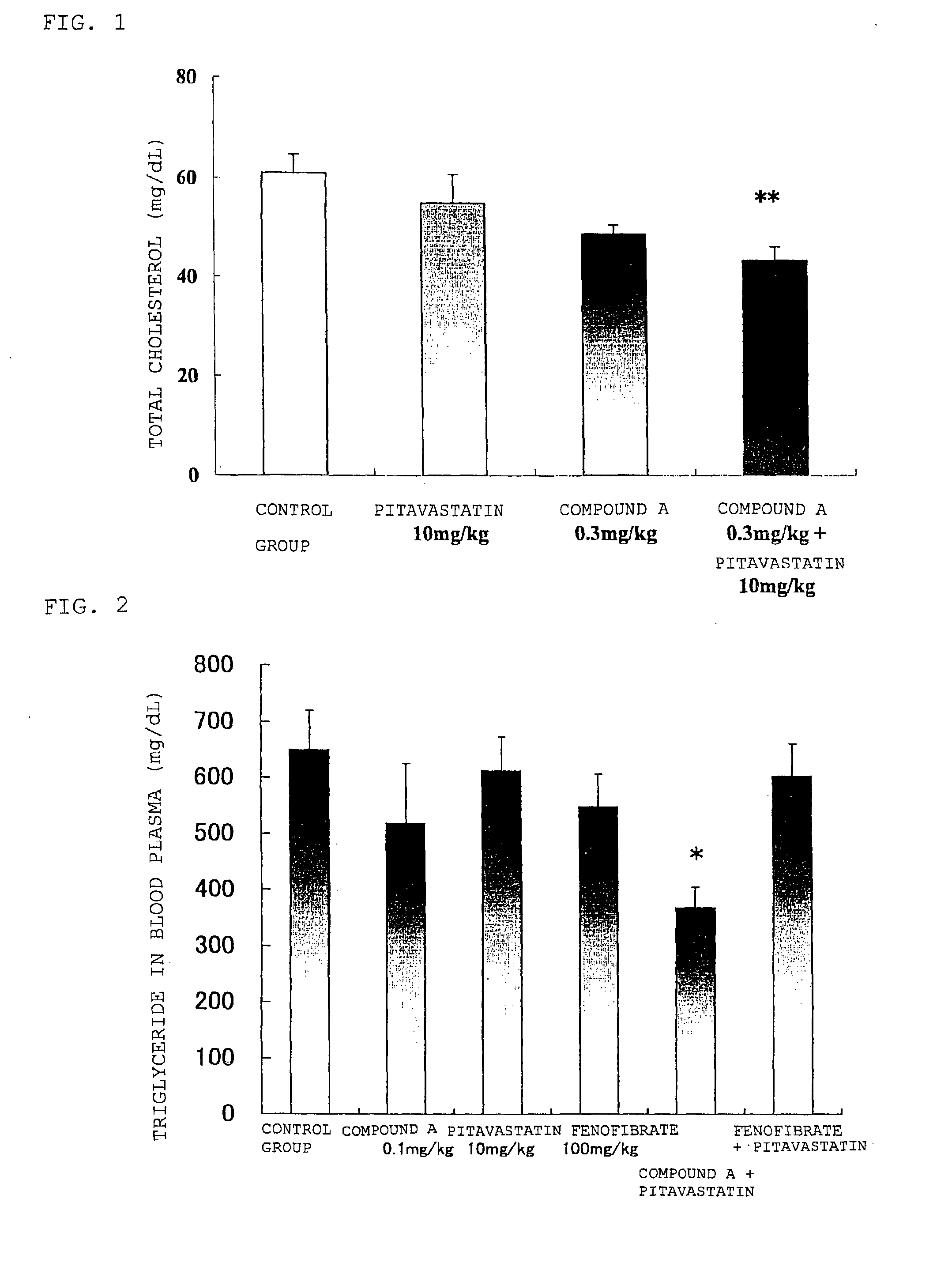
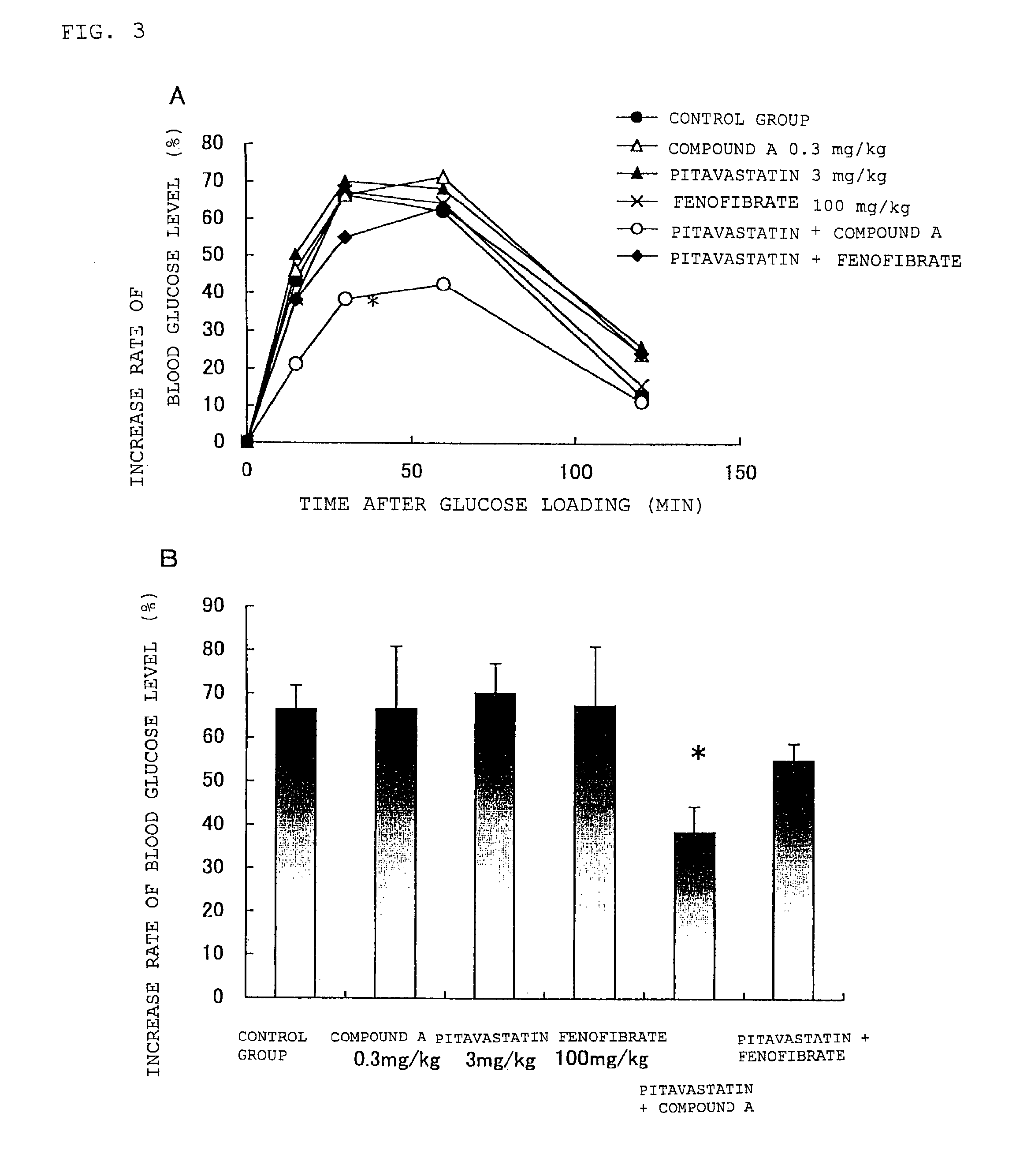
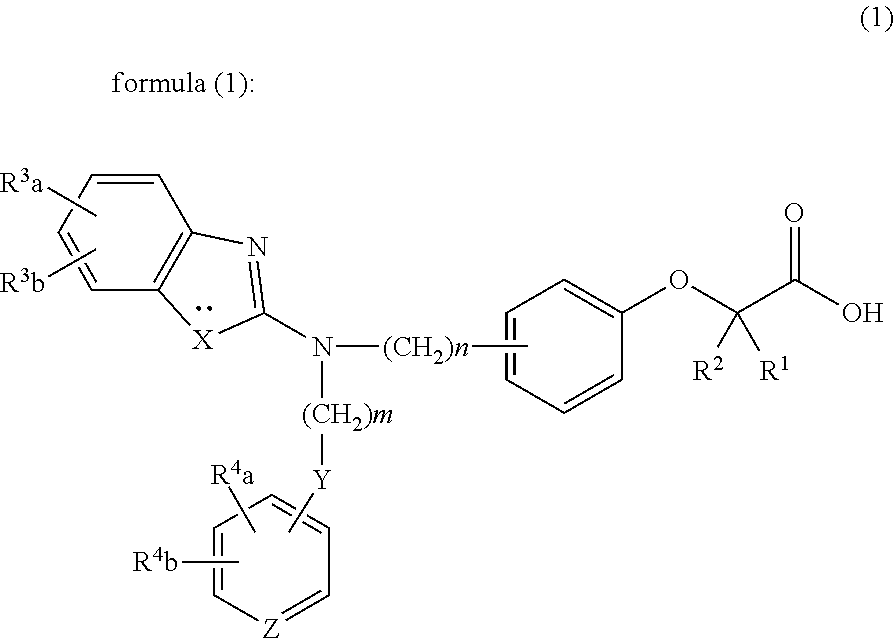
Prophylactic and/or therapeutic agent for hyperlipidemia
The present invention provides a therapeutic agent for hyperlipidemia having an excellent effect of lowering the cholesterol and triglyceride level in blood plasma.The present invention relates to a prophylactic and / or therapeutic agent for hyperlipidemia, a prophylactic and / or therapeutic agent for obesity or diabetes mellitus, and a prophylactic and / or therapeutic agent for metabolic syndrome, each agent including a compound represented by the formula (1), or a salt thereof, and a statin, particularly pitavastatin, in combination.
Owner:KOWA CO LTD
Pitavastatin calcium tablet and preparation method thereof
PendingCN114042052AImprove stabilityHigh dissolution rateMetabolism disorderInorganic non-active ingredientsAdhesivePitavastatin calcium
The invention discloses a pitavastatin calcium tablet which comprises a tablet core and a coating layer coated outside the tablet core, and the tablet core is prepared from the following components in parts by weight: 1-3 parts of pitavastatin calcium; 90 to 100 parts of a filling agent; 2-3 parts of a protection agent; the total amount of 15-20 parts of an internally added disintegrating agent and an externally added disintegrating agent; 150 to 200 parts of an adhesive; and 2-3 parts of a lubricant. The content uniformity of the tablet can be improved, the stability and the dissolution rate of the pitavastatin calcium tablet are effectively improved, and long-term storage is facilitated.
Owner:HAINAN HUALON PHARM
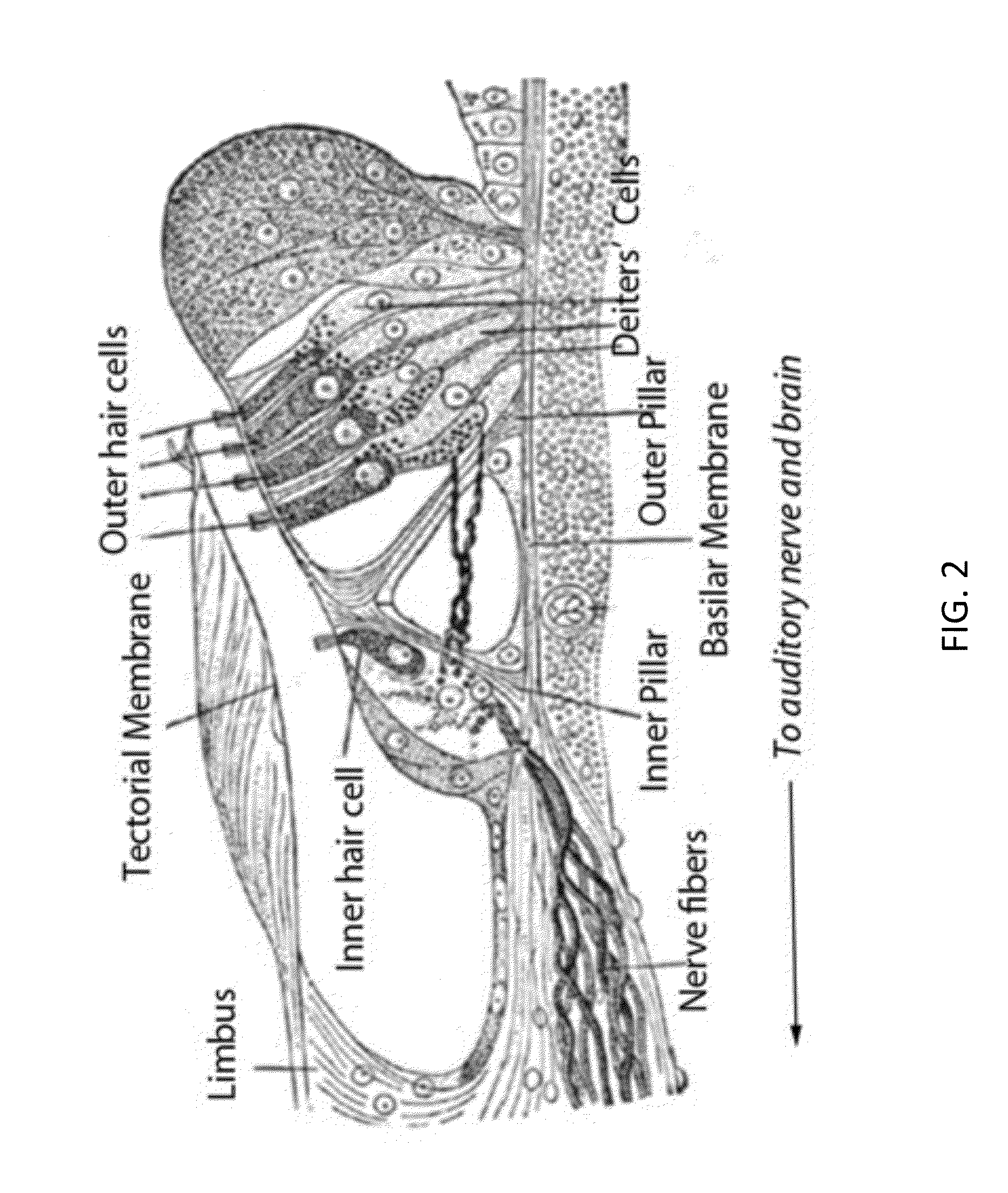
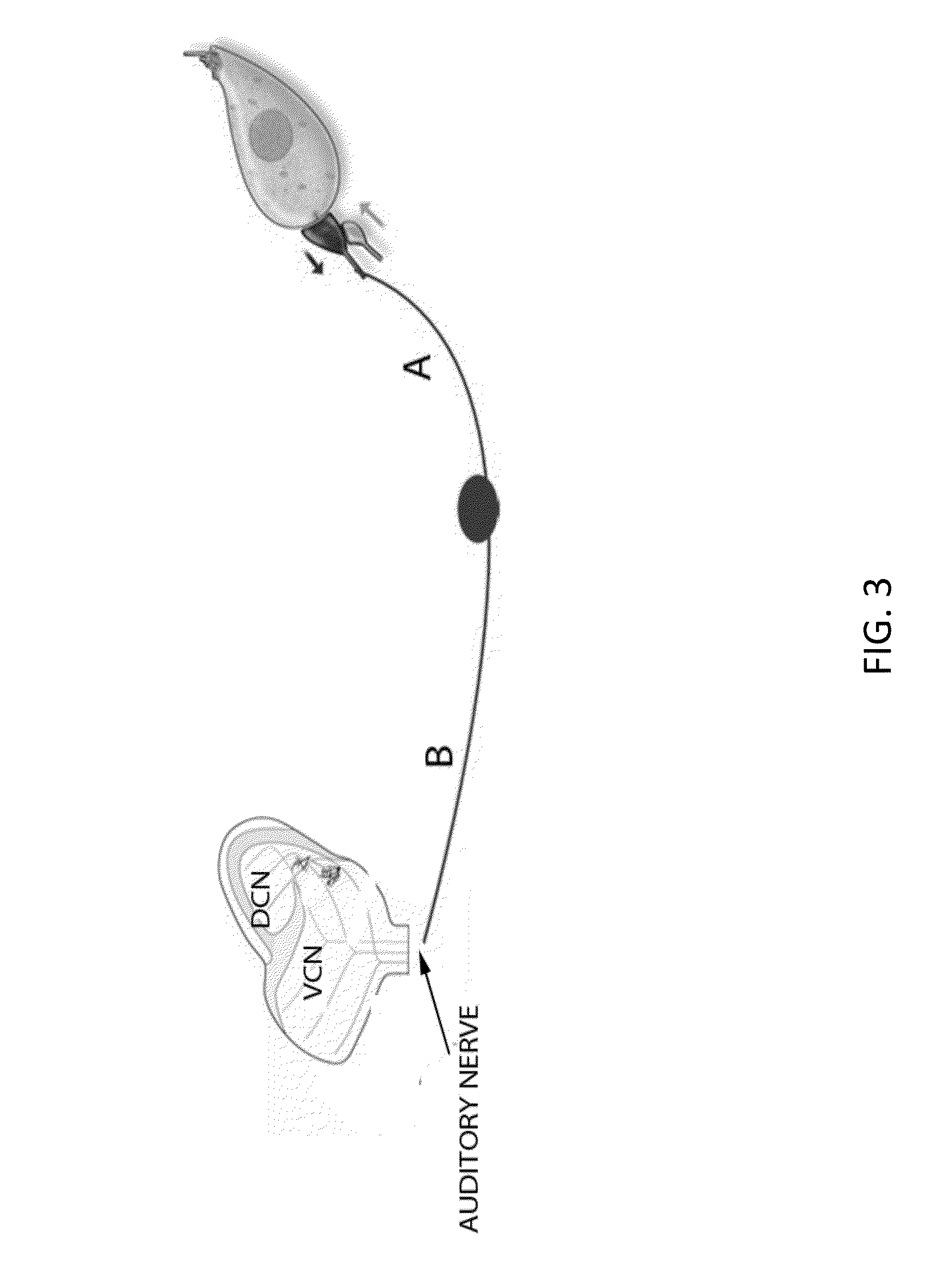
Local cochlear application of statins for stimulating neurite regrowth in the cochlea
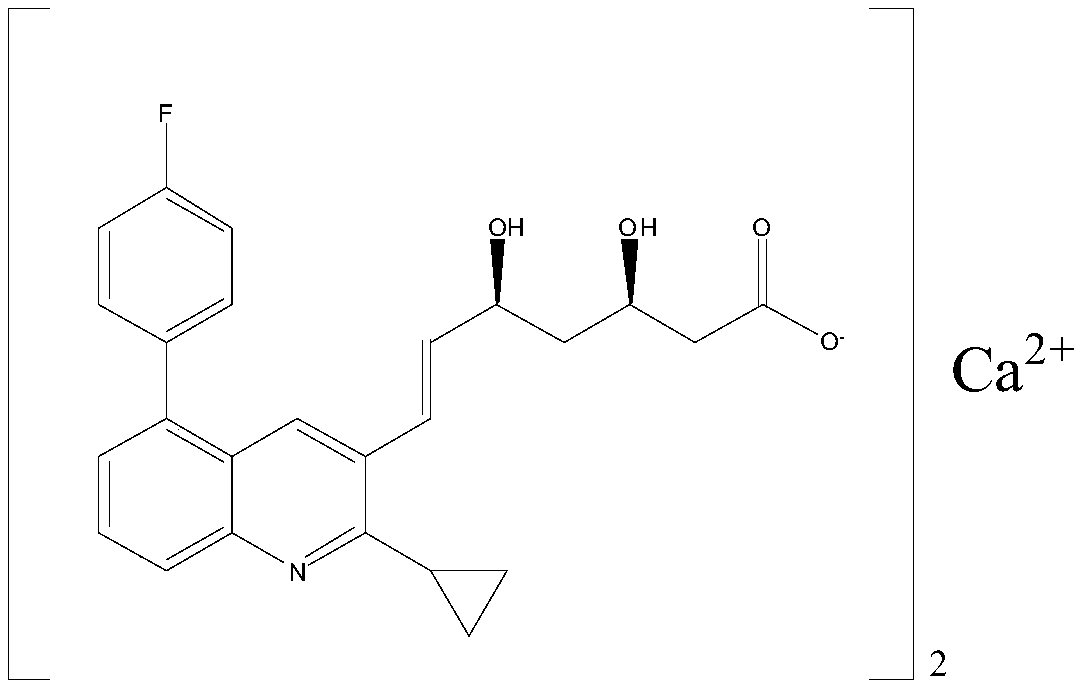
ActiveUS20160022663A1Preventing and treating hearing lossBiocideOrganic chemistryPitavastatinStatine
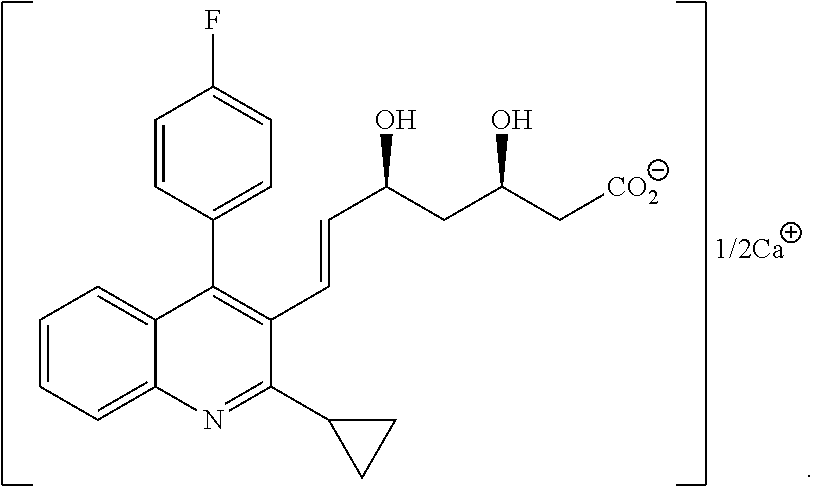
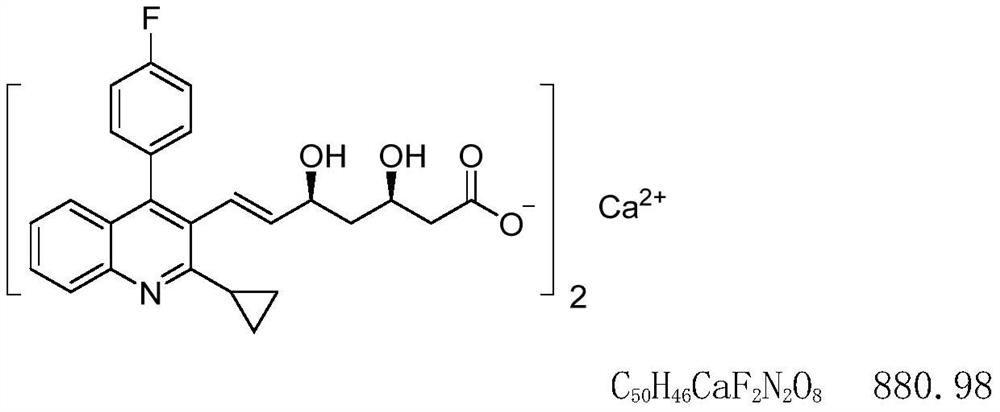
Statin compositions are disclosed for stimulating neurite growth from spiral ganglion neurons in the inner ear, as well as methods and kits for preventing damage to or treating damage of auditory neurons and / or hair cells of the cochlea following acoustic or toxic insult. An exemplary statin for these methods and kits includes Pitavastatin having the compound formula (VIII):
Owner:NORTHWESTERN UNIV
Method for synthesizing pitavastatin calcium intermediate by micro-channel reactor
ActiveCN111533688AConvenient storage and transportationReduce usageOrganic chemistryChemical/physical/physico-chemical microreactorsHydroxylamineHydroxylamine Hydrochloride
The invention discloses a method for synthesizing a pitavastatin calcium intermediate through a micro-channel reactor. The pitavastatin calcium intermediate is ethyl 7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dyhydroxyl-6-heptylate. The preparation method comprises the following steps: dissolving hydroxylamine hydrochloride into a mixed solution of water, alcohol and acetone; then, adding tert-butyl 6-[[(1E)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-vinyl]-2,2-dimethyl-1,3-dioxane-4-acetate, dissolving the added substance, filtering the obtained solution, feeding the solution toa micro-channel reactor preheating module, introducing the preheated reaction liquid into a reaction module, collecting the reaction liquid flowing out of a cooling module, and performing extraction and purification to obtain pitavastatin tert-butyl ester. The method provided by the invention has the advantages of small organic reagent dosage and high yield.
Owner:江苏星诺医药科技有限公司 +1
Preparation method of pitavastatin calcium intermediate
InactiveCN112175007AEasy to handleHigh yieldPhosphorus organic compoundsChemical compoundPhysical chemistry
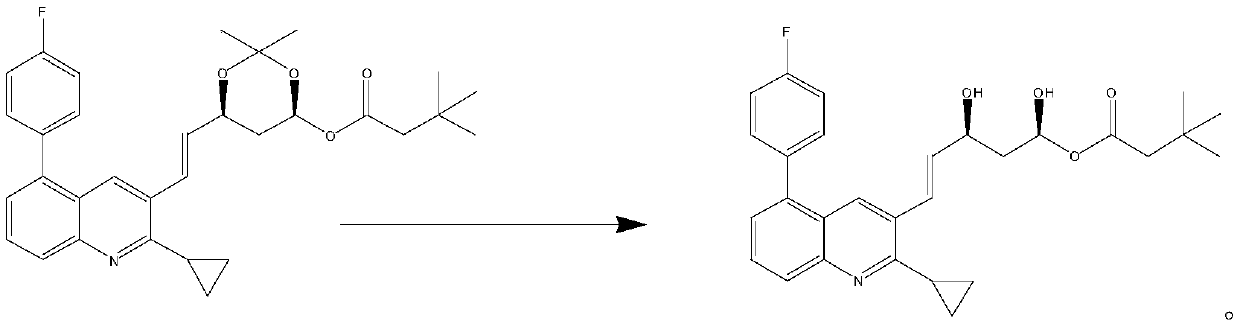
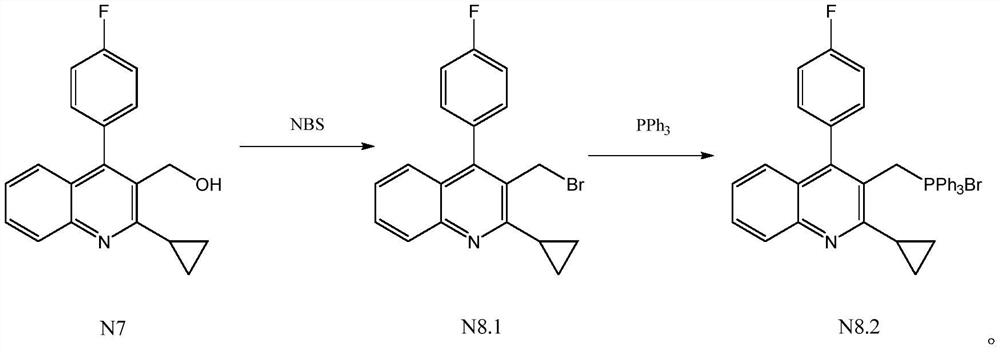
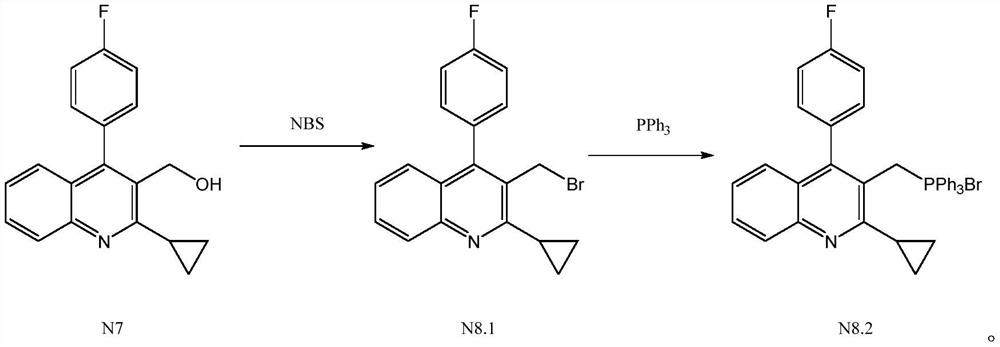
The invention relates to a preparation method of a pitavastatin calcium intermediate, which comprises the following steps: (1) under the protection of nitrogen, carrying out chemical reaction on compounds N7 and NBS and a solvent at 30-80 DEG C to prepare an intermediate product compound N8.1, and (2) adding triphenylphosphine into the reaction solution obtained in the step (1), heating to 60-120DEG C for reaction, cooling to 0-20 DEG C for stirring crystallization after the reaction is finished, and filtering to obtain a compound N8.2, wherein the specific synthetic route is as follows. Thepreparation method has the advantages of mild reaction conditions, no generation of a large amount of bromine-containing and phosphorus-containing wastewater in the reaction process, no influences ofacids and bases on the intermediate product compound N8.1, and difficult decomposition, so the yield of the target product is high and reaches 90% or above, the purity is high and reaches 99% or above, and the product has the advantages of simple post-treatment, greenness and environmental protection, and suitableness for industrial production.
Owner:江苏阿尔法集团福瑞药业(宿迁)有限公司
New method of pitavastatin calcium key intermediate
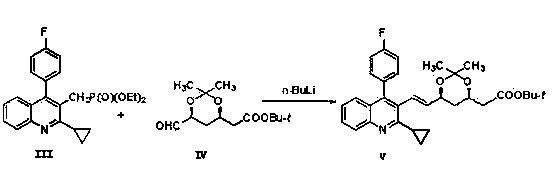
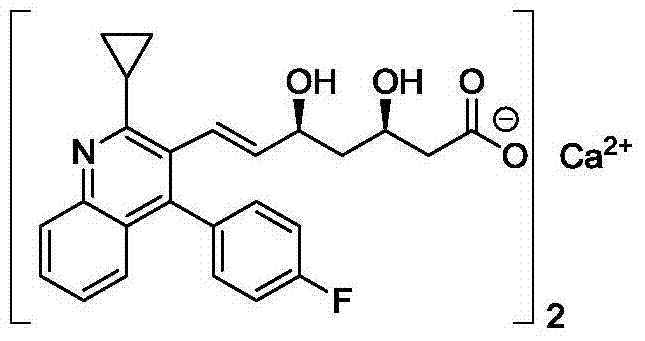
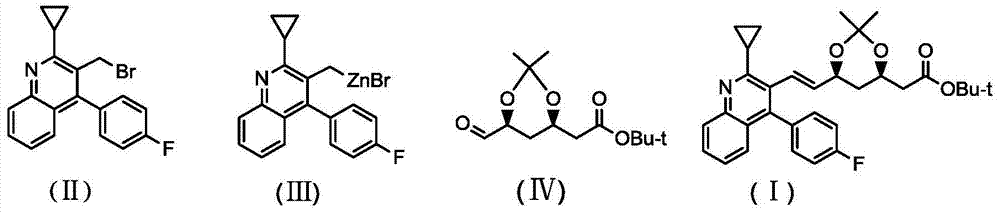
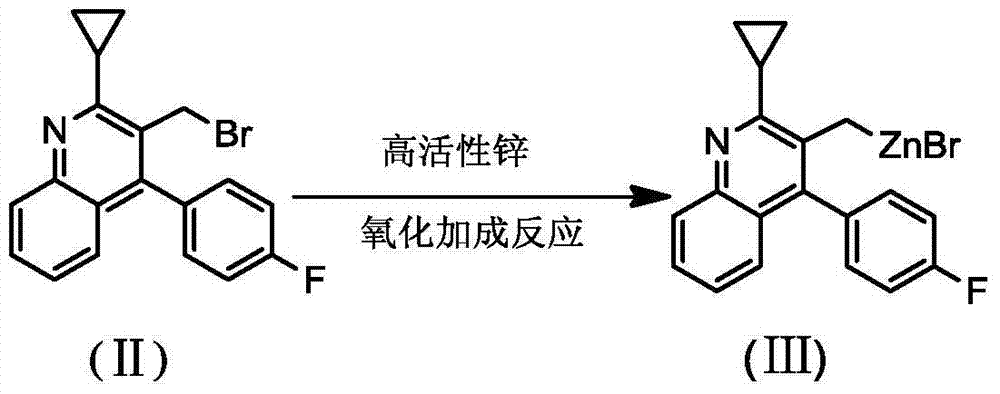
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a preparation method of a pitavastatin calcium key intermediate. The method comprises a step of preparing an organic zinc reagent by using high-activity zinc and 2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline methylene bromide. The organic zinc reagent generates a Witting reaction with (3R, 5S)-6-oxo-3,5-dihydroxyl-3,5-O-isopropylidene tert-butyl caproate to generate (3R, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolone-3-yl]-3,5-dihydroxyl-3,5- O-isopropylidene-6-tert-butyl heptenate which is recrystallized to obtain the pitavastatin calcium key intermediate. The synthesis method provided by the invention has the characteristics of high purity of E construction, simple process, high yield and the like.
Owner:北京华禧联合科技发展有限公司
Medicinal composition containing rosuvastatin or pitavastatin and B vitamin series
ActiveCN100502870CGood curative effectImprove compliancePill deliveryCapsule deliveryVascular endotheliumCerebro vascular disease
The invention relates to a pharmaceutical composition containing rosuvastatin or pitavastatin compounds and B vitamins; wherein the content of rosuvastatin or pitavastatin compounds is 1-40 mg, and the content of B vitamins is 0.1-50 mg . The invention also provides the application of the pharmaceutical composition in the preparation of medicines for preventing, treating and delaying atherosclerosis and related cardiovascular and cerebrovascular diseases. The invention has the advantages that the curative effect of the statin lipid-lowering drugs can be improved, the vascular endothelial protection and anti-atherosclerosis aspects are superior to the statin lipid-lowering drugs, the side effects of the statin lipid-lowering drugs are reduced, and the toxicity is not increased, In addition, it can make it convenient for patients to take medicines and reduce medical expenses.
Owner:SHENZHEN AUSA PHARM CO LTD
Method for producing pitavastatin calcium
ActiveUS10676441B2Promote safe productionHigh yieldMetabolism disorderOrganic chemistry methodsPhysical chemistryEngineering
Owner:API CORP (JP)
Analysis method for quantitatively detecting nitrogen and oxygen impurities in pitavastatin calcium
The invention discloses an analysis method for quantitatively detecting nitrogen and oxygen impurities in pitavastatin calcium. The analysis method adopts a high performance liquid chromatography, and performs qualitative and quantitative analysis on the nitrogen and oxygen impurities in the pitavastatin calcium by screening the detection wavelength, the mobile phase pH, a buffer salt type, gradient elution conditions, the sample injector temperature, the column temperature and the sample injection amount, and methodological verification is carried out. According to the method, nitrogen and oxygen impurities in the pitavastatin calcium can be effectively separated, qualitatively and quantitatively detected, and the analysis method is high in specificity and sensitivity, has a good linear curve in a low concentration range, is not influenced by personnel and instruments, has high repeatability and accuracy, and is stable and reliable.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Hypolipidemic medicament and preparation method thereof
ActiveCN111249248AImprove stabilityIncrease productivityMetabolism disorderInorganic non-active ingredientsCelluloseAluminum magnesium silicate
The invention provides a hypolipidemic medicament and a preparation method thereof. The hypolipidemic medicament comprises the following components in parts by mass: 2 parts of pitavastatin calcium, 2-2.4 parts of magnesium aluminum silicate, 0-0.5 part of montmorillonite, 90-100 parts of lactose, 16-20 parts of low-substituted hydroxypropyl cellulose, 1-3 parts of hydroxypropyl methylcellulose, 0.4-0.8 part of magnesium stearate, 0-1 part of copovidone S630 and 4-5 parts of a film coating premix (stomach-soluble type). According to the invention, montmorillonite and covidone S630 are added into auxiliary materials of pitavastatin calcium tablets, so that stability of the pitavastatin calcium tablets in a storage process is effectively improved, and medication safety is ensured. A simple mixing-tabletting-coating process is adopted, so that the defects of complex procedures and long time consumption caused by a conventional mixing mode of equal-amount progressive addition are avoided.The process is beneficial to improving production efficiency, and is convenient for commercial production.
Owner:北京阳光诺和药物研究股份有限公司
Preparation method of pitavastatin calcium oxide impurity
PendingCN113527269AQuality is easy to controlImprove medication safetyOrganic chemistryOrganosolvPitavastatin calcium
The invention relates to a preparation method of pitavastatin calcium oxide impurities, which comprises the following steps: by taking pitavastatin ester as a raw material, reacting with an oxidizing agent in an organic solvent to obtain an oxide of the pitavastatin ester, reacting with an inorganic alkali aqueous solution, and further hydrolyzing to obtain a salt of the oxide. The method can be used for qualitative and quantitative detection of impurities, and is beneficial to quality control and medication safety of pitavastatin.
Owner:SHANGHAI JINGXIN BIOLOGICAL MEDICAL +1
Method for detecting pitavastatin calcium intermediate and impurities
PendingCN114295748AImprove accuracyHigh sensitivityComponent separationBulk chemical productionFluid phaseSilanes
The invention provides a method for detecting pitavastatin calcium intermediates and impurities, which adopts high performance liquid chromatography to detect and analyze the pitavastatin calcium intermediates and impurities 1, 2, 3, 4 and 5, adopts octaalkylsilane bonded silica gel as a chromatographic column of a filling agent, adopts an ammonium acetate buffer solution as a mobile phase A, and adopts a mobile phase B to detect the pitavastatin calcium intermediates. Acetonitrile is used as a mobile phase B, and gradient elution is carried out. According to the method, known impurities can be accurately detected, the main peak of the intermediate and the peak of the known impurities can be effectively separated, and the method is a brand-new detection and analysis method beneficial to quality control of the pitavastatin calcium intermediate.
Owner:苏州正济药业有限公司
A kind of new preparation method of pitavastatin calcium intermediate
ActiveCN109867695BAvoid it happening againEasy to operatePhosphorus organic compoundsQuinolinePhenyl group
Owner:SHANGYU JINGXIN PHARMA +1
Medicine
InactiveUS20170246115A1Good disintegrationMaintain stabilityMetabolism disorderPharmaceutical non-active ingredientsCrospovidonesPitavastatin
The present invention provides a pharmaceutical product which includes a solid preparation comprising pitavastatin or a salt thereof, in which production of a lactone form thereof is suppressed.The pharmaceutical product is characterized by including a solid preparation comprising the following ingredients (A) and (B): (A) pitavastatin or a salt thereof; and (B) at least one member selected from the group consisting of carmellose and a salt thereof, crospovidone, and microcrystalline cellulose, and the solid preparation having a water content of 2.9 mass % or less, wherein the solid preparation is stored in a tight package.
Owner:KOWA CO LTD
A kind of refining method of pitavastatin tert-butyl ester
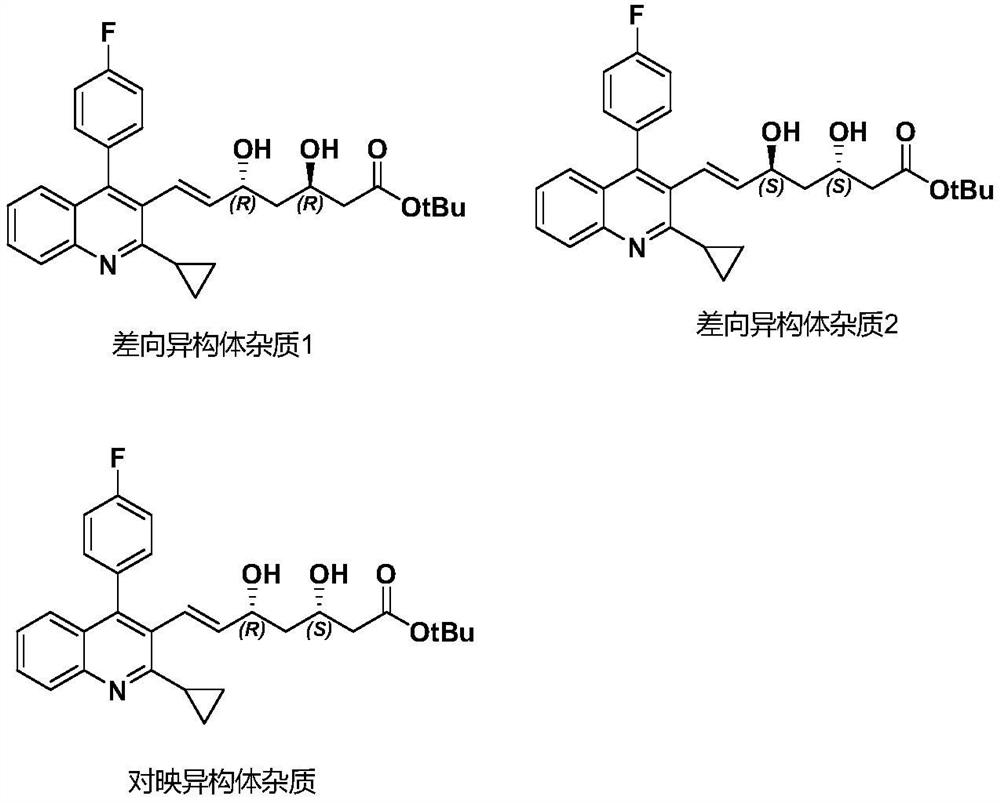
The invention discloses a refining method of pitavastatin tert-butyl ester, which comprises the following steps: cooling and crystallizing a mixed solvent in which pitavastatin tert-butyl ester raw materials are dissolved, filtering, and drying; wherein, the mixed solvent It is a mixture of methyl tert-butyl ether and non-polar organic solvent; the non-polar organic solvent is n-hexane, n-heptane, cyclohexane, n-pentane, cyclopentane, octane or isooctane. According to the refining method of the present invention, the isomer content in the obtained pitavastatin tert-butyl ester is low, and the refining effect is good. Epimer impurity 1, epimer impurity 2 and enantiomer impurity The contents are all below 0.05%. Moreover, the refining method of the present invention has high yield, low uncontrollability, simple operation, is suitable for large-scale industrial production, and can prepare high-purity pitavastatin calcium.
Owner:ZHEJIANG JINGXIN PHARMA
Solid oral pharmaceutical composition
PendingUS20220047547A1Increased riskIncrease heightInorganic non-active ingredientsPill deliveryPharmaceutical medicineBULK ACTIVE INGREDIENT
A solid oral pharmaceutical composition is disclosed, which comprises: a first active ingredient, which is pitavastatin or a pharmaceutically acceptable salt thereof; a second active ingredient, which is ezetimibe or a pharmaceutically acceptable salt thereof; and at least one excipient, including a diluent, a stabilizing agent, a disintegrant, a binding agent, a sweetener, a lubricant, a glidant, a flavor, a coloring agent or a combination thereof.
Owner:ORIENT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com