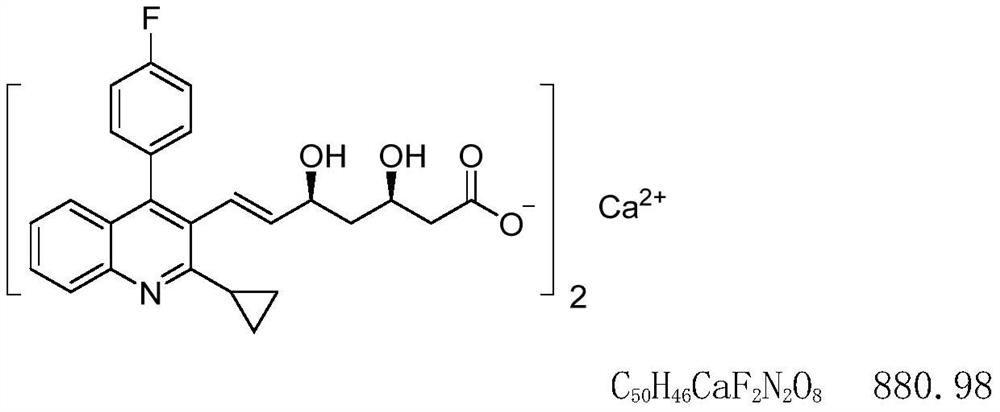
Analysis method for quantitatively detecting nitrogen and oxygen impurities in pitavastatin calcium
A technique for analyzing pitavastatin calcium and its analysis method, which is applied in the analytical field of quantitative detection of nitrogen and oxygen impurities in pitavastatin calcium, and can solve problems such as poor qualitative and quantitative detection and monitoring of nitrogen and oxygen impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
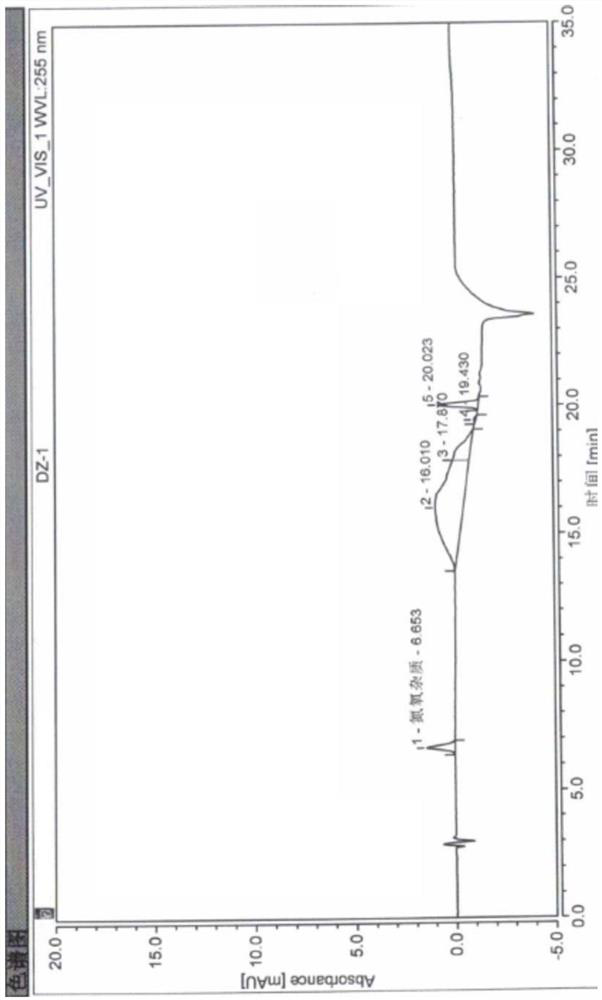
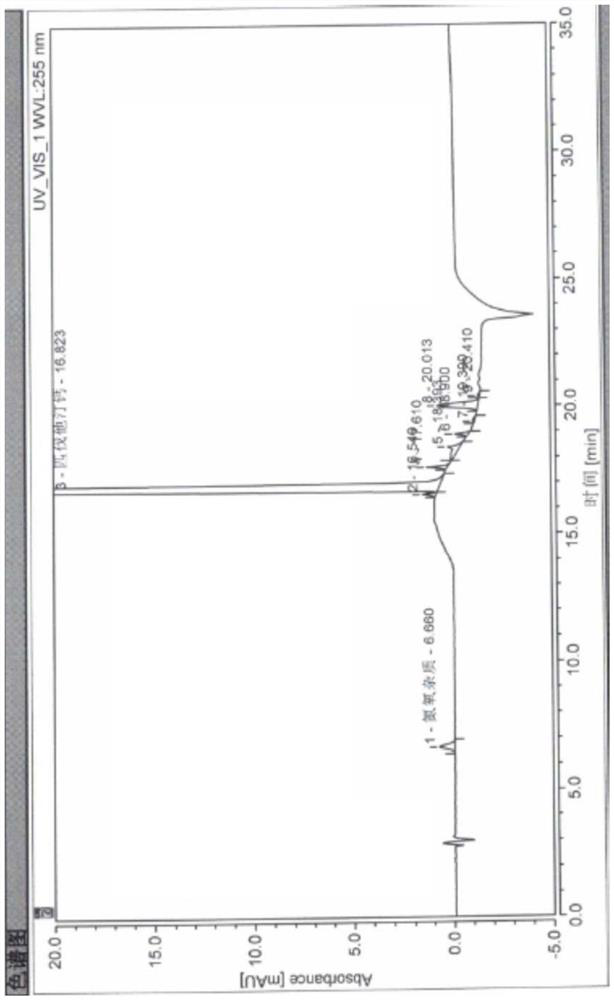
[0050] High performance liquid chromatography conditions:
[0051]The chromatographic column is a Welch Ultimate XB-C18 chromatographic column (250mm*4.6mm, 5μm), using dilute acetic acid buffer solution with a pH of 3.8 adjusted with sodium acetate solution and acetonitrile as mobile phase A and mobile phase B respectively, and the detection wavelength is 255nm. The flow rate is 1.0ml / min, the column temperature is 40°C, the injector temperature is 4°C, the injection volume is 10μl, and the gradient elution is performed according to the following table:
[0052] time A B 0 65 35 10 65 35 15 10 90 20 10 90 21 65 35 35 65 35
[0053] Sample preparation:
[0054] Pitavastatin calcium control solution: Weigh the reference substance, and dilute to the mark with a diluent to obtain a pitavastatin calcium control solution containing about 0.5 mg of pitavastatin calcium per 1 ml.
[0055] Pitavastatin calcium test solution: take th...
Embodiment 2
[0066] High performance liquid chromatography condition is the same as embodiment 1.
[0067] Sample preparation: use the blank mobile phase as the blank solvent for detection.
[0068] Experimental operation: Take 10 μl of blank solvent and inject, record the chromatogram.
[0069] The blank solvent selected by the inventors has been used to study the present invention. In the mobile phase chromatogram, there are no significant interference peaks near the peak positions of the main peak pitavastatin calcium and nitrogen and oxygen impurities. It is proved that the blank solvent does not interfere with the present invention.
Embodiment 3
[0071] High performance liquid chromatography condition is the same as embodiment 1.
[0072] Sample preparation:
[0073] Detection limit solution: a solution with a signal-to-noise ratio of nitrogen and oxygen impurities of about 3:1.
[0074] Limit of quantitation solution: a solution with a signal-to-noise ratio of nitrogen and oxygen impurities of about 10:1.
[0075] Test operation: Take 10 μl each of the detection limit solution and the quantification limit solution to inject samples respectively, and record the chromatogram. The inventor verified the detection limit and quantitative limit of nitrogen and oxygen impurities, and the results are shown in Table 2.
[0076] Table 2 Detection limit, limit of quantitation test result
[0077]
[0078] It can be seen from Table 2 that the finally determined detection limit and quantitative limit of nitrogen and oxygen impurities are very low, which proves that the nitrogen and oxygen impurities in pitavastatin calcium of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com