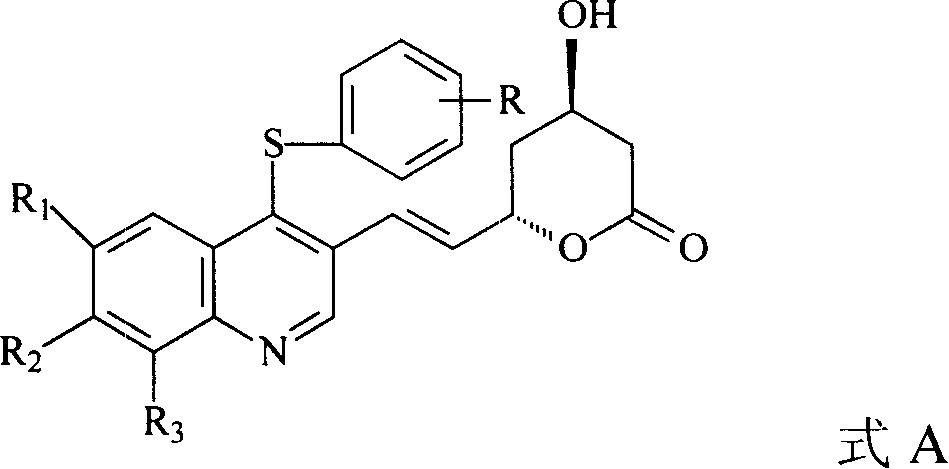
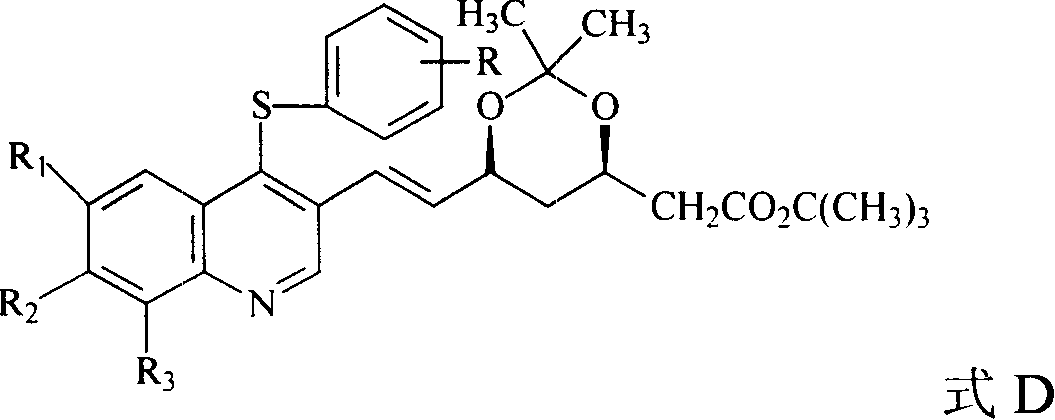
Quinolines compounds and their intermediates, preparation method and application
A compound, quinoline technology, applied in the field of medicinal chemistry synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of 4-chloro-6,7,8-trifluoroquinoline-3-carboxylic acid ethyl ester
[0048] 59.0 g of ethyl 4-hydroxy-6,7,8-trifluoroquinoline-3-carboxylate and POCl 3 500ml was refluxed for 8 hours. Atmospheric pressure evaporation to remove POCl 3 (Recoverable and applicable), the resulting residue was transferred to a vigorously stirred mixture of ice and water, solid NaHCO 3 Alkalinize to pH 7-8, filter with suction, and recrystallize from toluene to obtain 41.3 g of the product, yield 65.6%, mp: 110-112°C.
[0049] 4-hydroxy-quinoline-3-carboxylic acid ethyl ester, 4-hydroxy-6-fluoro-7-chloroquinoline-3-carboxylic acid ethyl ester and 4-hydroxy-7-chloroquinoline-3-carboxylic acid ethyl Esters are raw materials, and 4-chloro-quinoline-3-carboxylic acid ethyl ester, 4-chloro-6-fluoro-4,7-dichloroquinoline-3-carboxylic acid ethyl ester and 4,7- Dichloroquinoline-3-carboxylic acid ethyl ester.
Embodiment 2
[0050] Example 2 Preparation of 4-substituted phenylthio-quinoline-3-carboxylic acid ethyl ester (E1~4)
[0051] 4-Chloro-quinoline-3-carboxylic acid ethyl ester 8.0g (34mmol), 4-fluorothiophenol 5.2g (41mmol) and Et 3 N 6.9g (68mmol) was stirred in THF at room temperature for 30min. The insoluble matter was filtered off, the mother liquor was concentrated, and the mixed solvent (toluene and petroleum ether) was recrystallized to obtain 10.0g 4-(4-fluorophenylthio)quinoline-3- Ethyl carboxylate (E2).
[0052] According to the above method, 4-fluorothiophenol was replaced by thiophenol, m-methoxythiophenol and p-isopropylthiophenol respectively to obtain 4-phenylthio-quinoline-3-carboxylic acid ethyl Ester (E1), ethyl 4-(4-methoxyphenylthio)-quinoline-3-carboxylate (E3) and 4-(4-p-isopropylphenylthio)-quinoline-3- Ethyl carboxylate (E4). Yield, melting point (Mp.) and 1 H-NMR identification results are shown in Table 1.
Embodiment 3
[0053] Example 3 Preparation of ethyl 7-chloro-4-substituted phenylthioquinoline-3-carboxylate (E5-8)
[0054] With 4,7-dichloroquinoline-3-carboxylate ethyl ester as raw material, by the method for embodiment 2, respectively with thiophenol, 4-fluorothiophenol, m-methoxythiophenol and p-cymene Thiophenol was reacted to obtain compound E5~8 respectively, yield, melting point (Mp.) and 1 H-NMR identification results are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com