Preparation method of pitavastatin calcium intermediate
A technology of pitavastatin calcium and intermediates, applied in the field of drug synthesis, can solve the problems of affecting the total yield of products, product instability, consumption of large alkaline water, etc., and achieve simple post-processing of products, simple post-processing, and avoiding decomposition Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
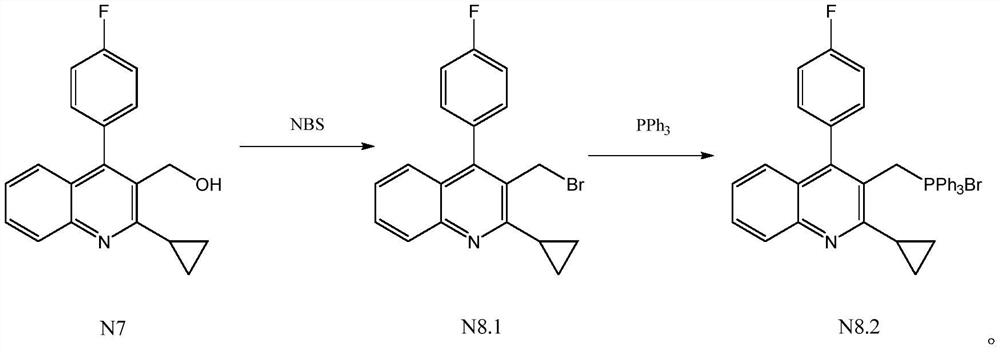
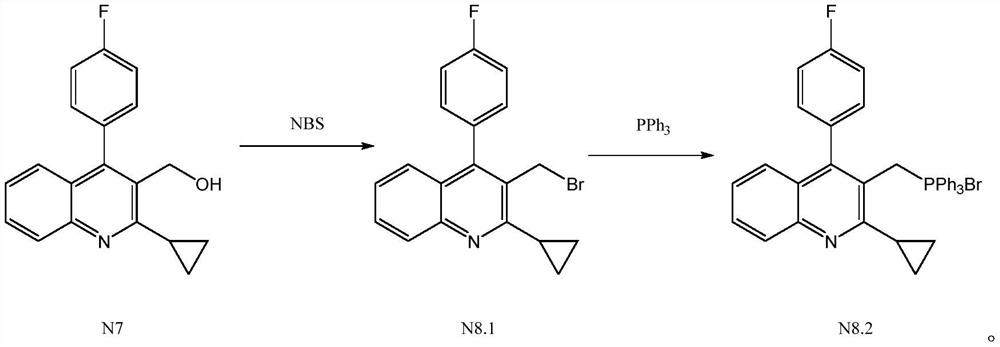
[0025] Add 40ml of toluene, 10g (0.034mol) 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol (N7) and 7.28g (0.041mol) NBS in the reaction flask, under nitrogen protection Under certain conditions, stir and raise the temperature to 50°C, and keep the temperature for 6 hours to prepare the intermediate product compound N8.1, without separation, directly add 10.29g (0.039mol) triphenylphosphine to the obtained reaction solution, stir and heat up to 70°C , keep the reaction for 6 hours, then lower the temperature to 0°C, stir and crystallize for 1 hour, centrifuge, and dry to obtain 19.75g of the product [[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl ]Methyl]triphenylphosphorous chloride (N8.2), yield 93.66%, purity after HPLC detection: 99.46%.
Embodiment 2
[0027] Add 100ml ethylbenzene, 10g (0.034mol) 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol (N7) and 10.92g (0.061mol) NBS in the reaction flask, under nitrogen protection Under the condition of stirring, the temperature was raised to 80°C, and the heat preservation reaction was carried out for 4 hours to prepare the intermediate product compound N8.1, without separation, 17.88g (0.068mol) triphenylphosphine was directly added to the obtained reaction solution, and the temperature was raised to 120°C with stirring. ℃, heat preservation reaction for 4 hours, then lower the temperature to 10 ℃, stir and crystallize for 2 hours, centrifuge, and dry to obtain 19.19g of product [[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline Base] methyl] triphenylphosphorous chloride (N8.2), yield 91.00%, purity after HPLC detection: 99.23%.
Embodiment 3
[0029] Add 50ml xylene (a mixture of o-paraxylene), 10g (0.034mol) 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol (N7) and 6.68g (0.0375mol) NBS, under the condition of nitrogen protection, stir and heat up to 60 ° C, and keep the temperature for 8 hours to prepare the intermediate product compound N8.1, without separation, directly add 11.62g (0.044mol) to the obtained reaction solution Triphenylphosphine, stir and heat up to 90°C, heat preservation reaction for 8 hours, then lower the temperature to 0°C, stir and crystallize for 1 hour, centrifuge, and dry to obtain 19.26g of product [[2-cyclopropyl-4-(4- Fluorophenyl)-3-quinolinyl]methyl]triphenylphosphine chloride (N8.2), yield 91.34%, purity after HPLC detection: 99.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com